Smart Biomaterials: Revolutionizing Drug Delivery
Smart biomaterials, sometimes referred to as responsive or stimuli-responsive biomaterials, are intricately engineered materials capable of responding to specific changes in their environment. This superior level of sophistication enables their potential to transform modern therapeutics, particularly in the field of drug delivery.
The Art of Drug Delivery
The process by which a medicinal or therapeutic treatment is deployed profoundly influences the ultimate effectiveness of that solution.
With crucial aspects such as release characteristics, absorption speed, and accuracy being impacted, it’s hardly surprising that researchers and drug manufacturers commit substantial resources to refine the drug delivery process. In addition to these complexities, a significant number of today’s medical interventions heavily depend on consistent human behavior, something that is notoriously unreliable.
With so many disadvantages having the potential to severely undermine the successful outcome of the therapy, there is an immense requirement, driven by a fusion of both need and convenience, for biomaterials that operate autonomously and intelligently, effectively eliminating the need for human micromanagement.
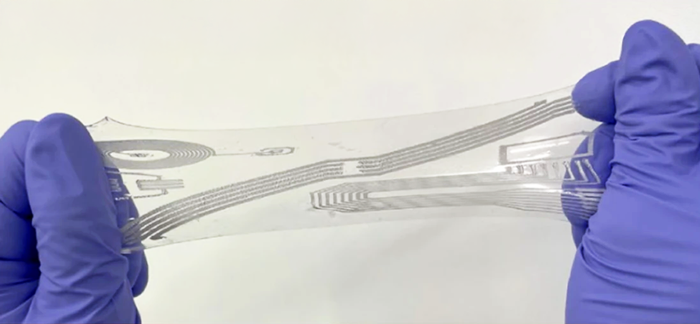
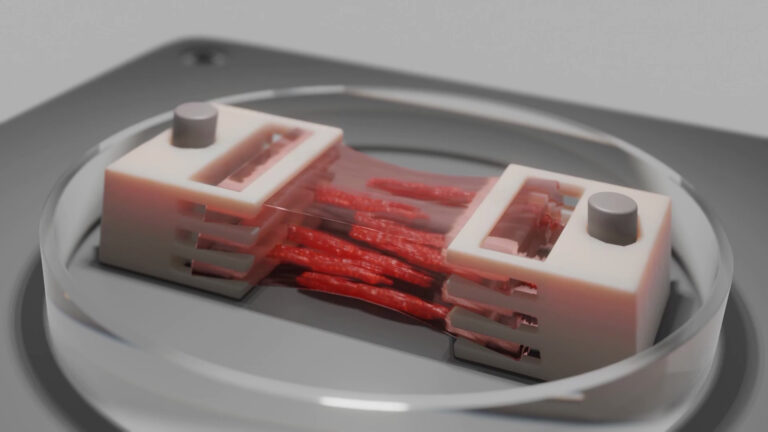
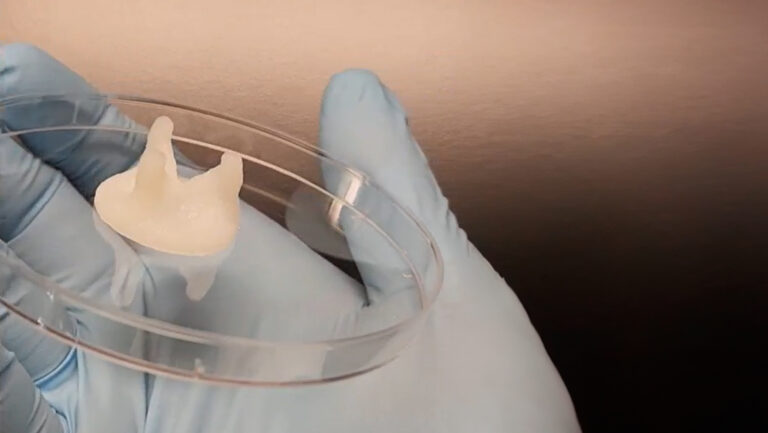
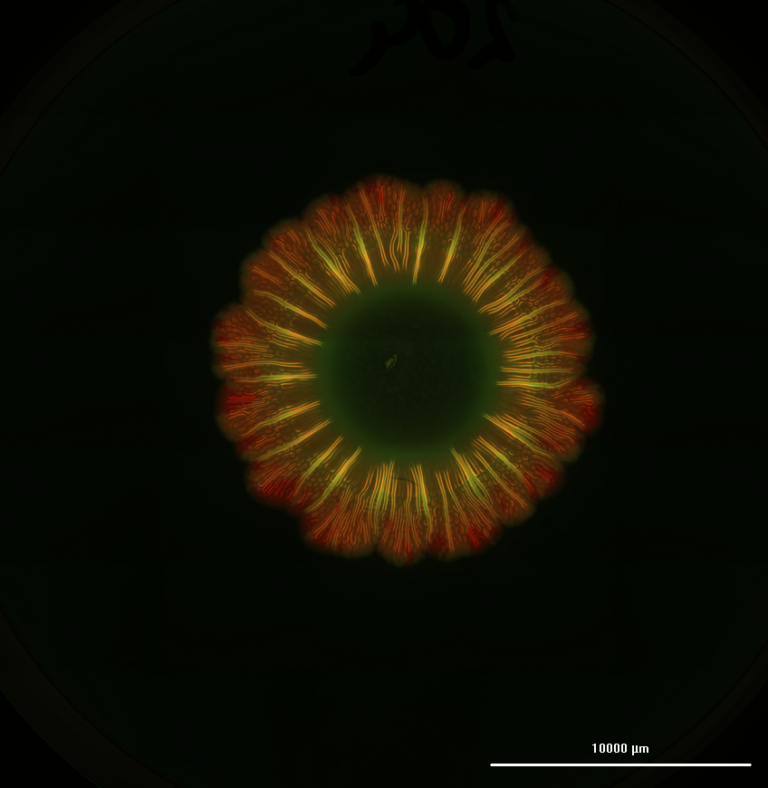
Professor Jeff Bates and his team at the University of Utah are taking this challenge head on. The team focuses on polymeric formulations that exhibit analyte sensitivity, enable triggered responses, are suitable for drug deliverability or ideally a combination of these behaviors. The teams goal is to convert these formulations into hydrogels or bioinks such that they can be bioprinted, leveraging the advantages of 3D bioprinting. In particular, the team is focusing on conditions that impact changes in fluid levels compared to homeostatic levels as can be seen in the eye or buccal cavity.
“There is a huge demand, based on a combination of necessity and convenience, for materials to be self-reliant and autonomous (smart) without the need for human intervention”
– Ashwin Velraj, PhD Candidate in Professor Bates’ lab
Multiple materials & Multiple modalities
To improve the process by which a treatment is deployed, the Bates group is thinking outside the box; pushing the limits of conventional bioprinting by combining multiple materials and bioprinting modalities.
The team leverages extrusion printing through the BIO X and DLP based bioprinting with the LUMEN X. This novel approach of combining two different bioprinting techniques, gives them a unique ability to capture specific geometries that better recapitulate in vivo environments. Specifically, the power of the BIO X is used to print multi-platform, multilayered and multicomponents constructs, while the LUMEN X and the inherent high resolution provided by light-based printing is used to prototype finer details in photosensitive hydrogels and subsequent upscaling strategies.

What comes next in smart biomaterials?
Professor Bates and his team see tremendous potential for bioprinting to usher in an era of personalized healthcare delivered at the point of care. Essential to unlocking this advantage however and delivering the full power of smart biomaterials is ensuring their printability.
Professor Bates and his team see the next stage of their research focusing on fine tuning these functional polymers to ensure not only reproducible printing but optimizing the printing process to widen who can carry out these tasks, further enabling the possibilities of point of care treatments.

Ashwin Velraj, PhD Candidate in Bates’ lab, spoke more in detail of what they are seeking to accomplish:
“We wish to lay out the barebones of what it takes to print materials across different printing technologies, by overcoming the limitations of polymeric formulations that may arise due to their innate properties. Based on our current work, we look forward to getting to a stage where multilayered platforms consisting of diagnostic and therapeutic modalities can be rapidly printed with minimal training or knowledge of polymer science, to bring this technology as an easy tool for customized disease detection and trigger responsive drug delivery options.