Providing a new lease on life
Bioprinting pediatric heart valves that stand the test of time
Heart valve disease is a significant global health issue, affecting millions of people and contributing to a substantial number of cardiovascular-related deaths. Traditional mechanical replacements, while life-saving, present several challenges, especially over time. Most mechanical valves do not grow or adapt with the patient’s body, leading to the need for multiple invasive surgeries. This not only poses inherent risks but also significantly impacts the patient’s quality of life. Issues such as blood clots, calcification, and severe inflammation further complicate the situation. These challenges become even more critical in pediatric cases, where conventional solutions, while still life-saving, may reduce life expectancy by up to 50%.
Professor Savoji, Arman Jafari (a PhD student in his lab) and a dedicated team at the University of Montreal have employed bioprinting and their BIO X6 to address these challenges and develop a method to bioprint heart valve replacements.
The Savoji Lab's Innovative Approach
Recognizing the potential of bioprinting, the Savoji Lab embarked on a mission to develop a material that would enable high-fidelity printing without a support bath. By eliminating the need for a support bath, they avoid the challenges associated with batch-to-batch variations of such support materials, and simplify the biofabrication process. Another requirement of this material was to impart the mechanical properties required for a heart valve, namely the ability to withstand the load of blood flow and return to its original shape consistently.
The Savoji Lab’s groundbreaking solution involves a material composed of Polyvinyl Alcohol (PVA), Gelatin (Gel), and K-carrageenan (CG). This combination offers a unique blend of properties that make it suitable for bioprinting heart valves. Notably, the material can be cyclically frozen and thawed prior to printing to providing it with properties that support complex printing while simultaneously imparting the necessary micro architecture for cell adhesion and proliferation, ensuring stability and functionality of the construct over time.
In order to perfect their bioink formulation, the Savoji lab carried out extensive tests, scanning through material properties like rheological characteristics, microstructure, mechanical characterization, and swelling and degradation, to name a few. With their BIO X6, they had an open-source system with the required resolution and temperature capabilities to enable these experiments.

Adv Funct Materials, First published: 10 October 2023, DOI: (10.1002/adfm.202305188)
Proving the biocompatibility of the bioink
After settling on the ideal composition of PVA, Gel, and CG that provided high printability, limiting swelling, and perhaps most importantly, a young’s modulus close to in vivo heart tissue, they were able to progress into determining biocompatibility. This was done through a number of cell-based studies, including cytotoxicity, hemocompatibility, and finally, in vivo performance following subcutaneous implantation into mice.
After the 3 month long in vivo experiments, the team concluded that this novel bioink caused no negative inflammatory reactions in the mice, and all 9 of the mice survived. The subsequent histological evaluations demonstrated that these scaffolds promoted the development of collagen, increasing by 25% after 4 weeks. The team also observed that after 12 weeks, the native tissue had begun to integrate with the explant, implying an acceptance from the host tissue. Perhaps most importantly, however, the retrieved tissues showed no signs of calcium deposits, implying that they had avoided calcification, one of the most significant problems with commercially available heart valves.
The feasibility of realistic 3D printed heart valves
With the material properties ironed out and the biocompatibility proven, the team turned to the feasibility of printing realistic heart valves that would withstand the demands of a complex organ system like the cardiovascular system.
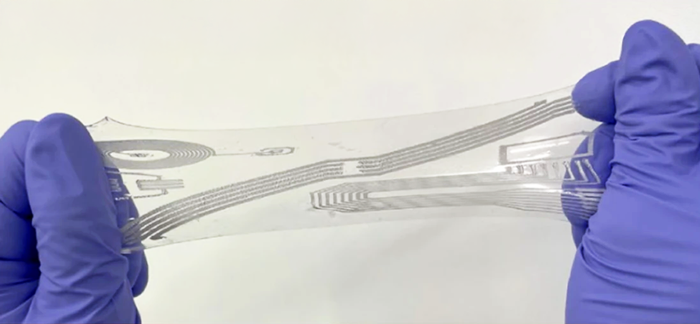
Taking mCT scans, Prof Savoji and his team were able to create physiologically relevant 3D models that were effortlessly loaded on to the BIO X6 for the final test of their material. Thanks to the high resolution of the bioprinter, and the inherent material properties, the team was able to print heart valve models to scale, without the need for a support bath while maintaining performance in line with FRESH printed models that were previously published. To further push the boundaries of their research, the team connected these bioprinted valves to a pulse duplicator system, which created heartbeat-like waves. The bioprinted valve responded as expected in vivo, with the leaflet opening and closing.
µCT scan layer-by-layer rendering of the valve in the transverse plane – University of Montreal
The Savoji Lab’s research, facilitated by the BIO X6 bioprinter, represents a significant leap forward in the quest for more effective and sustainable heart valve replacements. The innovative material developed addresses the shortcomings of traditional mechanical solutions, offering the promise of personalized, integrated, and growth-capable heart valves. As bioprinting technology continues to advance, it holds the potential to revolutionize not only cardiac care but also the broader landscape of regenerative medicine and personalized healthcare.
Further reading
The full paper from the Savoji lab is published in the prestigious Advanced Functional Materials journal and can be read here: https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202305188
For more information about the Savoji lab, visit their website: Savoji Lab
Learn more about the BIO X6 3D bioprinter here: BIO X6
More Customer Spotlights

Stretchable Electronics through 3D Bioprinting

Innovative Silk Bioink for 3D Bioprinted Bone Marrow Tissue Models

Empowering Highly Motivated Researchers

Smart Biomaterials: Revolutionizing Drug Delivery

Advancing Lung Cancer Research

3D Bioprinted Models for Cochlear Implant Evaluation
Bioprinting biofilms with GelMA

Making a Difference in Patient Care with 4D Bioprinting

Stretchable Electronics through 3D Bioprinting
Professor Steve Park from Korea Advanced Institute of Science and Technology (KAIST) is heavily involved in the study of stretchable electronics, and his lab has published multiple ground-breaking publications on both soft electronics and biosensors. In this customer spotlight, we take a deeper look at how the BIO X6 3D bioprinter has helped Professor Park and his research group realize their novel ideas, expanding their research. We will also highlight the compelling results of their work.

Bioprinted Skeletal Muscle Tissue with a Perfusable Microchannel Network
Skeletal muscle tissue (SMT), the body’s largest organ by mass, plays a vital role in movement, posture, breathing, overall physiology and energy homeostasis. However, genetic disorders, diseases, injuries, and aging can impair this essential organ, significantly impacting health and quality of life. Traditional 3D culture systems have struggled to replicate the complex structure and functionality of native muscle tissue, particularly at larger, centimeter scales. Using advanced bioprinting technologies, Dr. Miriam Filippi and her group from the Soft Robotics Laboratory (led by Prof. Robert Katzschmann at ETH Zurich) have developed biohybrid SMT constructs that effectively mimic native muscle tissue.

Innovative Silk Bioink for 3D Bioprinted Bone Marrow Tissue Models
There is a rising need of platelet units, which is primarily sourced from healthy donors. However, the natural production of platelets coupled with the brief shelf life does not meet the demand by the healthcare industry. Prof. Alessandra Balduini and her team at the University of Pavia are taking this challenge head-on by initiating the EIC Transition SILKink project, which aims to produce a groundbreaking platform that combines the use of natural silk and 3D bioprinting to recreate the environment of the human bone marrow where platelets are produced.

Empowering Highly Motivated Researchers
We have interviewed Takaaki Arahira, the Associate Professor of the Department of Information Networks, Faculty of Management and Information Sciences at Kyushu Institute of Information Sciences.





