Bioprinted Skeletal Muscle Tissue with a Perfusable Microchannel Network
Skeletal muscle tissue (SMT), the body’s largest organ by mass, plays a vital role in movement, posture, breathing, overall physiology and energy homeostasis. However, genetic disorders, diseases, injuries, and aging can impair this essential organ, significantly impacting health and quality of life. Traditional 3D culture systems have struggled to replicate the complex structure and functionality of native muscle tissue, particularly at larger, centimeter scales.
Using advanced bioprinting technologies, Dr. Miriam Filippi and her group from the Soft Robotics Laboratory (led by Prof. Robert Katzschmann at ETH Zurich) have developed biohybrid SMT constructs that effectively mimic native muscle tissue.
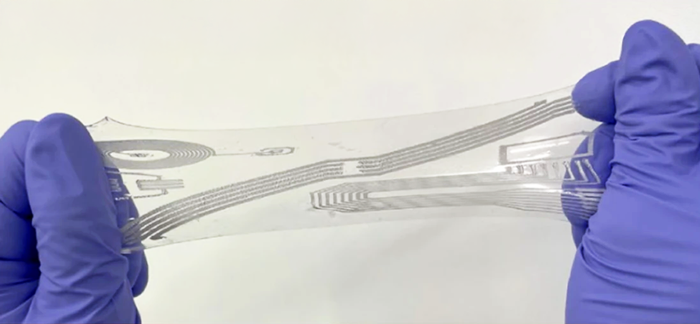
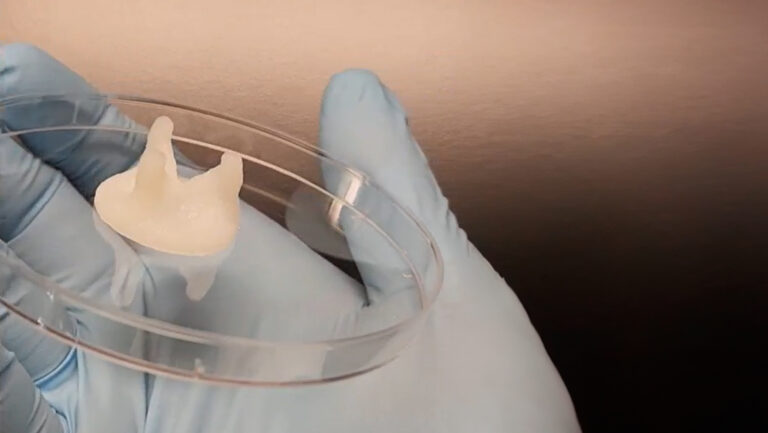
By formulating specialized bioinks for extrusion-based bioprinting, the team created constructs with finely distributed microchannels that model the functional perfusion of native muscle structure on the cm-scale. These microchannels ensure efficient nutrient and oxygen delivery, which is critical for cell survival. The integration of synthetic anchors provided the necessary mechanical tension for aligned muscle fiber growth and proper tissue maturation.
The team employed multi-material extrusion-based bioprinting on the BIO X6 to realize a perfusable, biohybrid SMT, with the entire construct being fabricated in one go. The SMT constructs featured multi-layered tissue with internal microchannels measuring 200 µm in diameter. These constructs were composed of GelMA-Alginate bioink seeded with C2C12 myoblast cells. The channels were created using a sacrificial bioink (Pluronics) that was removed post-printing by lowering the temperature below its gelation point. Additionally, an anchor structure made of PEGDA and Pluronics was incorporated to secure the construct to the culture template that provides the mechanical tension needed for tissue development.
Watch this video to learn more about the group's process
Video produced by Moritz Hocher
Bioprinting as a Tool to Fabricate Complex Models
From the very beginning, Dr. Filippi knew that achieving her goals would not be possible with other hydrogel manipulation techniques. Bioprinting was chosen because it offers the precision required to create interconnected cavities according to a predefined design—an essential aspect for their complex model. Multi-material extrusion bioprinting allowed for a resolution of 300-400 microns, which was sufficient to achieve the desired architectural features, namely the finely spread perfusion channel network and the stable interface between the synthetic and biological components of the system. Thus, fabrication via bioprinting ensured an adequate perfusion level to prevent cell death and sufficient bioassembly stability for completing the tissue maturation process.
Developing a bioink suitable for muscle tissue development
The team developed and optimized a bioink with suitable mechanical properties for bioprinting, muscle tissue development, and stable co-assembly with synthetic structures.
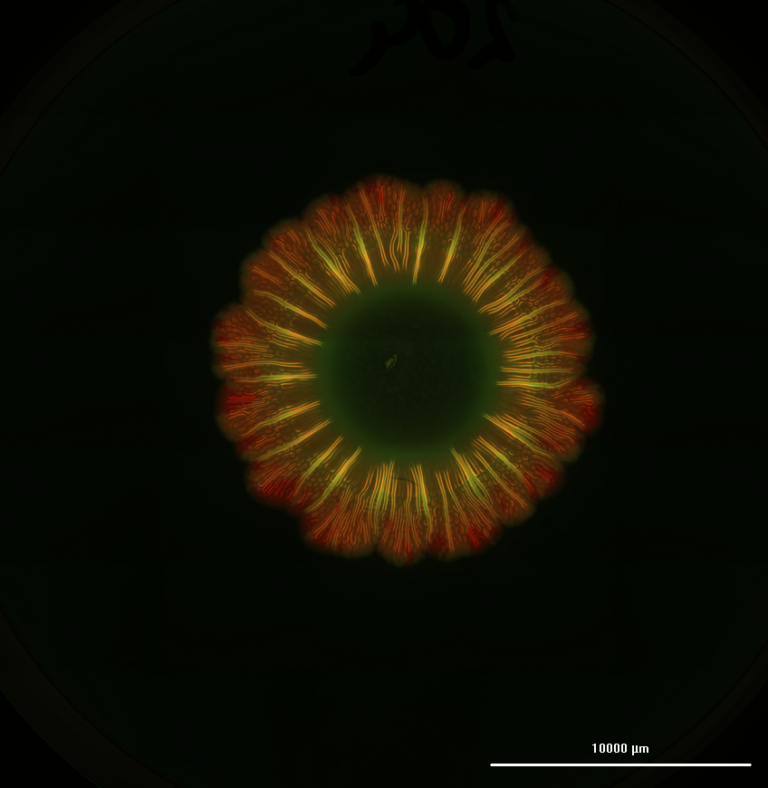
The biohybrid SMT remained functional for two weeks. Patterning perfusable networks within the constructs reduced hypoxic regions and provided contact guidance to the growing myocytes, promoting the formation of unidirectionally aligned muscle fibers.
Assessing construct perfusability for in vitro drug testing
The team evaluated their perfusable bioprinted construct for drug testing by analyzing drug distribution and release within channeled versus unchanneled matrices. Dye tests showed that channels enabled rapid liquid convection within minutes. Further studies demonstrated that intratissular drug distribution was significantly influenced by the channels, highlighting the importance of fluid convection and diffusion in the construct. These findings support that vessel-like channels and a perfusable design are crucial for creating a biomimetic model of vascularized muscle tissue, enhancing its relevance for pharmaceutical research.
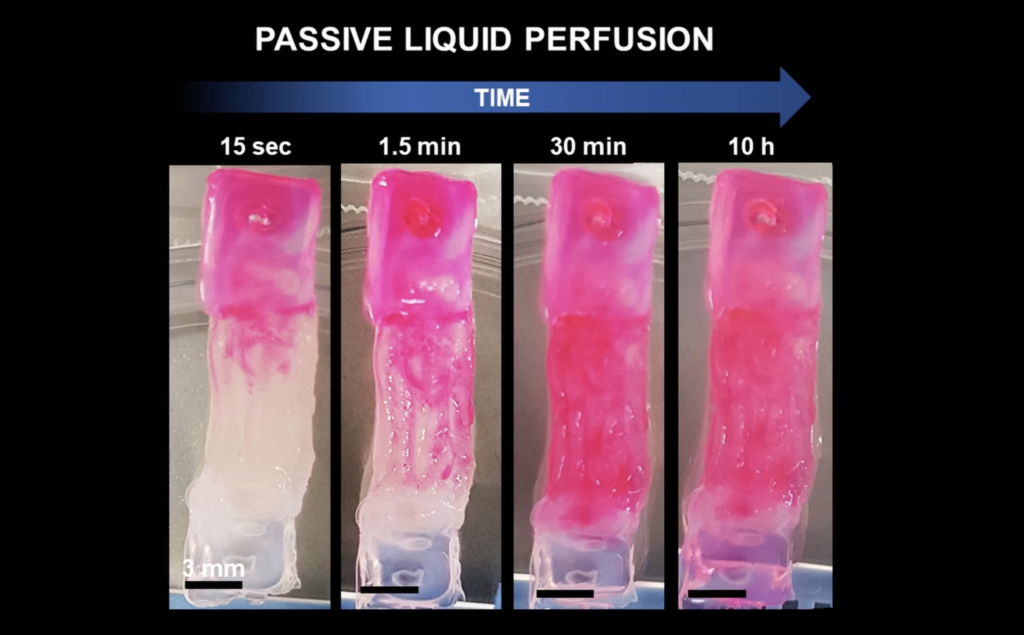
Liquid perfusion through channeled engineered muscle tissue (Filippi et al., Advanced Healthcare Materials, 2023).
The Bioprinting Experience
The team created a model that wouldn’t have been possible with manual deposition or other biofabrication techniques. High-resolution biofabrication, offered by CELLINK’s BIO X6 bioprinter was needed to achieve the project goals. Working with multiple bioinks in a single fabrication round was challenging, however, the team found it useful that they could rely on CELLINK to provide the right technology and biomaterials so they could focus on the biofabrication step. Furthermore, the widespread use of CELLINK’s products facilitated collaboration and knowledge exchange with other labs.
Another aspect was the usability of the software.

Dr. Miriam Filippi
“The software is built in a very user-friendly way. Academic labs are quite dynamic, so it was very good that the know-how could be easily transferred. All the students in my lab get up to speed with it very quickly. So, if you have someone with expertise in the team, you can transfer it easily.”
Impact and Future Applications
Dr. Filippi and her team demonstrated that 3D extrusion-based bioprinting can engineer complex, multiphase constructs with living cells, biomaterials, synthetic materials, and perfusable networks. The team expects that multi-material bioprinting will advance muscle tissue engineering for biomedicine, nutrition, and biorobotics, addressing key challenges such as shape fidelity, structural stability, efficient liquid exchange, and prevention of hypoxic areas.
In conclusion, Dr. Filippi and her team’s innovative use of bioprinting technology represents a significant advancement in SMT engineering, offering new possibilities for medical, nutritional, and robotic applications.
Learn More
Publication:
M. Filippi, O. Yasa, J. Giachino, R. Graf, A. Balciunaite, L. Stefani, R. K. Katzschmann, Perfusable Biohybrid Designs for Bioprinted Skeletal Muscle Tissue. Adv. Healthcare Mater. 2023, 12, 2300151. https://doi.org/10.1002/adhm.202300151
Visit the lab’s website: https://srl.ethz.ch/research/BiohybridRobotics.html