Extrusion Bioprinting vs DLP Bioprinting, Explained
Extrusion bioprinting and DLP bioprinting are both 3D printing technologies focused on biofabricated constructs. From stiff bone to soft fat, and from miniscule capillaries to a full-sized brain… Our cells have an ability to form a myriad of tissue types. This is one of the most challenging aspects of tissue engineering, and a key reason why different bioprinting technologies exist. In order to reproduce the complexity of a human body, tissue engineers will have to harness a variety of technologies instead of a one-size-fits-all solution.
In this article we explain the differences between extrusion bioprinting and DLP bioprinting, as well as their advantages and disadvantages. If you want to have a deeper dive into the technologies we offer, see our extrusion bioprinters BIO X and BIO X6. We also highly recommend taking a look at our DLP bioprinters LUMEN X and BIONOVA X.
Continue reading for an in-depth comparison between the 3D bioprinting technologies where we will go through printing mechanics, resolution, geometry and material compatability.
Printing mechanics: the differences
Both extrusion bioprinting and DLP bioprinting begin with a computer-aided design (CAD) file. This is file is subsequently sliced into discrete layers, which the printers then build and stack to produce the 3D construct. The difference lies in how each printing method treats those layers.
Printing mechanics of extrusion bioprinting
With extrusion bioprinting, the more common technique in the bioprinting industry, a paste or fluid is loaded into a cartridge mounted on a gantry or robotic arm. This gantry then moves along Cartesian coordinates above the printbed, or surface, the object will be printed on. A mechanical force, commonly air pressure or a motor-driven piston, pushes the material through a nozzle to form a filament. By dragging the filament across the surface, the gantry traces the outline of the first layer. Then, as specified by the user, the gantry continues depositing filament layer by layer in an infill pattern to establish porosity and mechanical strength. This continues until the print is complete.
Printing mechanics of DLP bioprinting
With DLP bioprinting, we’re also using a layer-by-layer process. Instead of extruding material through a nozzle, a source of illumination treats each layer with a still image. This image is projected into a bath or droplet of light-sensitive liquid, which stimulates a chemical reaction that causes the liquid to cure where illuminated. Stacking these cured layers on a build platform produces the printed object.
Difference in printing resolution
The most common comparison when discussing printing techniques is the resolution – the smallest theoretically printable detail. The resolution is governed by a number of factors such as material and geometry, these are outside the scope of this article. This comparison of extrusion bioprinting resolution and DLP bioprinting resolution is based on the planar resolution along the X and Y axes.
Resolution of DLP bioprinting
In DLP bioprinting, the size of the projected pixel defines the smallest point of light that can be cured. This point of light is typically smaller than most extrusion nozzle diameters. In addition to this, the chemical reaction is more consistent in a DLP bioprinter. Together, these factors means that DLP bioprinting constructs can be both smaller, more intricate and a higher resolution than extrusion bioprinting. As an example, LUMEN X offers a feature resolution of 35 µm, and BIONOVA X a feature resolution of 10 μm.
Resolution of extrusion bioprinting
In extrusion bioprinting, it’s the nozzle diameter which dictates the diameter of the filament that can be extruded. After the filament leaves the nozzle of an extrusion bioprinter, nothing controls how the filament lays down and spreads other than gravity and friction. This results in some variability along the boundary of the filament. Even with a filament that is the same size as the smallest point-of-light from a DLP-based projector, an extruded print will look rougher than a DLP bioprinted construct given this variable spread.
The resolution can be improved with the user of embedded bioprinting techniques such as FRESH bioprinting. In short, this bioprinting technique involves using a LifeSupport™ bath which is specifically formulated to prevent hydrogel constructs from deforming during the printing process. In addition to reducing the variability (and thus increasing the resolution), it allows tissue engineers to print more complex structures. You can read more about the benefits of FRESH bioprinting in our dedicated blog.
Enhanced microfluidic applications in DLP bioprinting
Extruded prints are, essentially, a stacking of cylinders. This means the contact area between filaments is very small. Now stack these cylinders into a tubular shape, like that of a blood vessel. Similar to if you’re stacking perfectly cylindrical logs into a tube, the small contact area makes it difficult to ensure that the tube is water tight and strong enough to withstand the pressure of fluid flowing through it. You get most of the way there, but don’t quite make it.
This is where DLP bioprinting comes in. In DLP bioprinting we’re putting an entire layer of material on top of another layer, essentially gluing them from edge to edge, yielding significantly stronger, watertight structures. With stronger tubes and smoother sides, DLP bioprinting is well-suited for bioprinting lab-on-a-chip microfluidic devices, especially ones with complicated networks. Or ones that need to be imaged, distortion-free, under a microscope. DLP’s advantages when printing negative features like microfluidic networks also contribute to the technology’s ability to print complex positive features like latices.
Controlling porosity in extrusion bioprinting vs. DLP bioprinting
A)
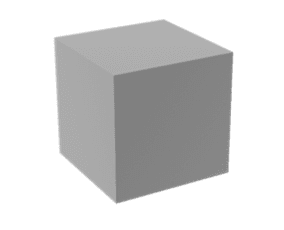
B)
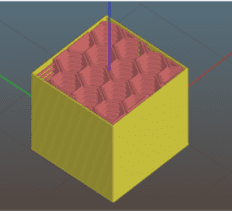
C)
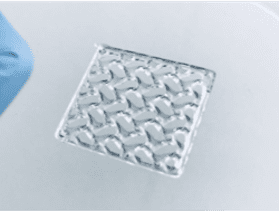
Figure 1. Extrusion 3D printing of a cube. A) CAD model of a cube, B) image of a slice showing perimeter (yellow) and infill (red) paths, C) the extruded cube.
While extrusion slicers focus on the outer bounds of an object, DLP-based SLA slicers capture the entire plane of a layer in a single image, operating with 100% infill, so that any porosity desired must be created in the original model. While this might pose upfront complications, many CAD suites can apply a lattice pattern to an object, such as the cube in Figure 2.
A)
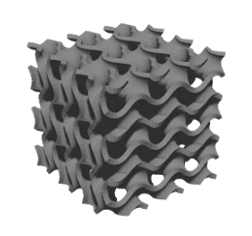
B)

C)
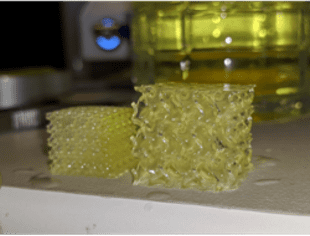
Figure 2. DLP-based 3D printing of a cube. A) CAD model of a cube with a gyroid structure, B) image of a slice that would be projected onto the photo-sensitive material, C) the printed gyroid cube (right).
Tissues, like bone, have porosities and geometries in three dimensions, so being able to generate and print a repeating lattice or randomized 3D structure will yield a scaffold that better mimics physiological tissue. The filament-by-filament extrusion-based approach has an inherent fragility that makes printing lattices, like the structure in Figure 2, nearly impossible. In addition, in extruded constructs, porosity exists vertically, while horizontal pores occur between layers and are therefore limited or nonexistent. Given the different strengths of each technique, users must consider the best suited method of bioprinting for their design. While extrusion offers a simplified design and print process that makes it well suited for labs with limited access to CAD software or just starting out, light-based bioprinting techniques are currently the best option for reproducing the smallest, most intricate structures of the human body. However, shape and size are only part of the tissue engineering puzzle.
Selecting the right biomaterials
Biomaterials is one of the most crucial aspects of tissue engineering, and are a key component as to why we need both DLP bioprinting and extrusion bioprinting in a workflow. Certainly, bioprinting organs would be far easier if organs were composed of only one biomaterial and one cell type. But properly recreating an organ means capturing the spatial arrangement of different cell types.
A bioprinter built on extrusion technology can array any number of cartridges on a gantry (at CELLINK we do up to 6 printheads), enabling the arrangement of different biomaterials and cells which, filament by filament precisely mimics the in-vivo environment.
In contrast, a DLP-based bioprinter is currently only one material and usually only one cell type. There have been multiple attempts to perform multi-material DLP printing in which the arrangement of the material or cell type doesn’t matter; however, these attempts usually involve slow, repeated cleaning steps, and run the disk of cross contamination between wash liquids or from one material to another.
Extrusion allows for multi-material bioprinting in addition to making it possible to print with a wider variety of materials. The simplicity of pushing fluids through a cartridge affords tissue engineers creativity and flexibility both before and after extrusion for liquefying then solidifying materials. The nearly infinite possibilities of extruded materials explains the variety of printheads available for the BIO X, allowing researchers to combine many materials and cell types in a single print. As the name implies, light-based bioprinting techniques, like the LUMEN X and BIONOVA X, use photo-sensitive materials and a light source to promote solidification. The photo-sensitive material must also have a low enough viscosity to flow into a droplet as the build platform moves into and out of the liquid during the print.
Combining extrusion bioprinting with DLP bioprinting
When viewed through the lenses of mechanics, resolution, geometry and material selection, there are numerous differences between extrusion and DLP bioprinters; however, the advantages of each make them perfectly complementary in many research environments. Extrusion offers multi-material printing from a vast palette of bioinks as well as simplified model designs, while DLP is capable of remarkable geometric complexity at high resolution and, depending on the material used, perfect clarity. Researchers might also combine the two techniques in a single experiment. For example, by extruding a mix of cells from the BIO X into a microfluidic chip printed on the LUMEN X. Whether you choose to add one or both to your workflow, you will find that CELLINK offers reliable, versatile bioprinting solutions.




