FRESH Bioprinting enables more complex geometries
FRESH Bioprinting, or Freeform Reversible Embedding of Suspended Hydrogels Bioprinting, was originally developed as a 3D bioprinting method to address the many limitations faced when using soft biomaterials for tissue engineering applications. FRESH bioprinting is performed by extruding bioinks into a LifeSupport™ bath specifically formulated to prevent hydrogel constructs from collapsing and deforming during the printing process. By incorporating ions, enzymes, pH buffers and more, a wide range of polymer crosslinking chemistries and gelation mechanisms can be supported within LifeSupport™.
FRESH has rapidly become the go-to bioprinting platform of choice for many tissue engineers. FRESH enables bioprinting with any soft gelling biomaterial at higher resolutions with no limitations on geometric complexity. FRESH can be integrated into the standard bioprinting workflow and implemented on an extrusion-based bioprinter like the BIO X™. FRESH drastically transforms bioprinting, allowing researchers to tackle the pressing challenges of complex tissue architecture and function. For example, FRESH bioprinting eliminates the need for tedious tasks of ink-specific print optimization, letting researchers skip to bioprinting truly 3D scaffolds and tissues.

FRESH bioprinting on a BIO X is as simple as putting a prepared dish of LifeSupport™ onto the print platform and dropping the printing needle into the center of the dish to begin fabricating complex geometries.
Explore promising research directions for FRESH bioprinting
1. Printing tissue-like complexity
The major appeal of FRESH bioprinting is its ability to create complex structures out of soft biomaterials. This capability lends easily to a potential world of FRESH-printed constructs with structural and compositional complexities closer to those of native tissues.
Every organ and tissue in the body comes with unique structural and compositional complexities that engineers have sought to recreate in biomimetic approaches. Examples like the heart or tendinous tissue, where tissue anisotropy (having directionality) is critical to tissue mechanical properties and function, highlight the importance of fabricating more physiologically relevant tissue structures. Most importantly, FRESH bioprinting is not limited to tissue architecture.
With FRESH, researchers have the opportunity to engineer constructs outside of the biological template. By identifying critical functional parameters for tissue function, researchers can now leverage existing tools such as computationally generated designs to optimize printed constructs for key features that contribute to the desired tissue functions, such as porosity, mechanical properties and construct-cell interactions.
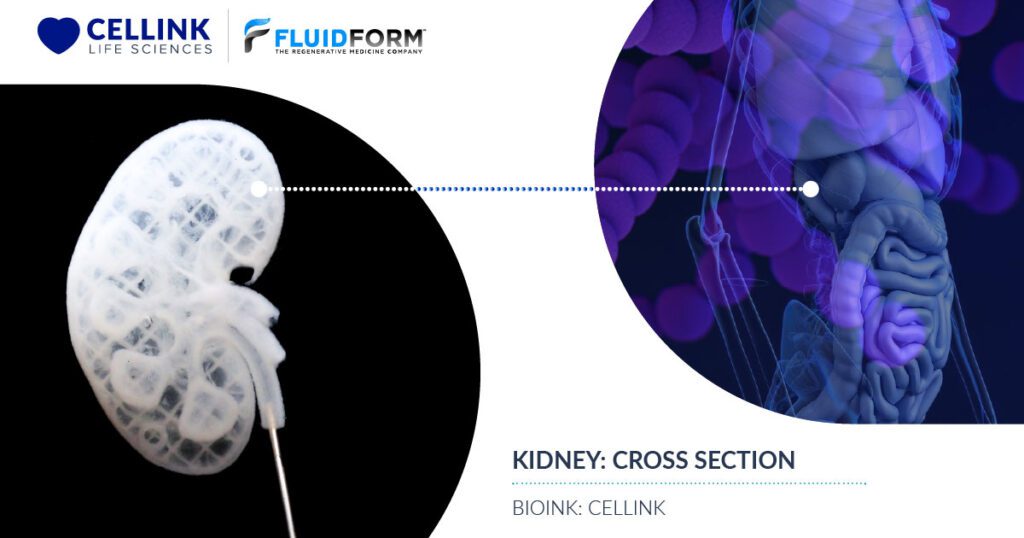
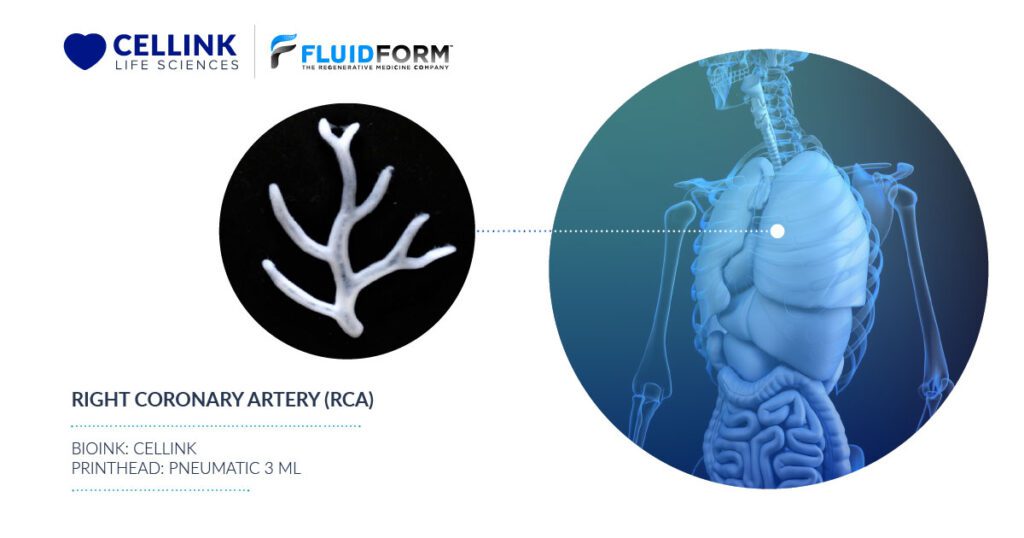
2. Creating Vascularized Tissue
For tissue engineering applications, FRESH bioprinting is one of the few technologies that allows freeform design and fabrication of multiscale vasculature networks. As tissue vascularization has captured the imagination of scientists and researchers for decades, the literature is rich with studies of neo-vascularization of implanted constructs, growth factor-induced vascularization and microfluidic devices with perfusable single channels, all of which are driven by the goal of one day being able to fully vascularize and keep alive a thick (>1 cm in all dimensions) piece of tissue.
While printed materials such as Pluronic F127 or gelatin have been used effectively as sacrificial materials to create lumen structures within a bulk casted matrix, the lumen diameters are often limited to the width of a single extrusion from a print nozzle.
FRESH instead enables the bioprinting of high-resolution features from the bottom up—features such as thin vessel walls and tortuous vasculature structures with variable diameters. With FRESH, it is now possible to fabricate complex and detailed vessel networks with control over lumen and wall thickness dimensions from the micrometer to millimeter length scales and with 3D branched, hierarchical vasculature derived from medical imaging files.
3. Multimaterial bioprinting
When printing multiple materials on a single hardware platform, researchers face many challenges.
First, the platform hardware must be designed to handle multiple toolheads, and the extrusion of different materials from each of these toolheads must be well coordinated with the software. The BIO X™ and BIO X6™ accomplish this with multiple toolhead systems and an easy-to-use software interface.
Secondly, and perhaps more importantly, when bioprinting multimaterial constructs, the hardware must enable the dispensing and gelling of multiple materials in a single container. This is no easy feat, considering the wide-ranging gelation requirements that exist among biomaterials. Unlike traditional 3D biofabrication methods, FRESH bioprinting allows for the simultaneous printing of multiple biomaterials that crosslink using ionic, chemical, photochemical, enzymatic and physical gelation, all within a single dish.
Multimaterial biofabrication is easier when printing into LifeSupport™ because enzymes, ions and chemicals that are compatible with gelatin can be easily mixed in to drive ink-specific gelation of the printed materials. For example, alginate, a commonly bioprinted material, can be printed into a support bath mixed with calcium ions, and collagen can be physically crosslinked when printed into a support bath that is buffered with a pH neutral buffer. These two distinct bioinks can be printed together in a multimaterial print by using a support bath that contains both a pH neutral buffer and calcium ions.

Learning more about FRESH Bioprinting
By combining the BIO X™ bioprinter with the FRESH Bioprinting method, researchers can unlock a new realm of print complexity and functionality previously only dreamed of.
Whether you’re an experienced researcher using bioprinting, or are interested in FRESH Bioprinting, don’t hesitate to contact us here.




