Redefining Personalized Medicine with Bioprinted Cartilage Grafts

Identifying the problem
CELLINK’s INKREDIBLE+™ bioprinter and Advanced BioMatrix’s LifeInk® 200 type I collagen were used in a groundbreaking nasal cartilage bioprinting study recently published in the Journal of Tissue Engineering. Here, co-authors Professor Adetola Adesida, head of the eponymous lab at the University of Alberta, and fifth year PhD candidate Xiaoyi Lan discuss how they 3D bioprinted human nasal cartilage cells into grafts that remained stable after subcutaneous implantation in a murine model.
According to Dr. Adesida, about 40% of skin cancer patients develop nasal lesions. After surgical removal, it is notoriously difficult to reconstruct the affected nasal cartilage with grafts taken from the patient’s ribcage, for example. In a 2014 study, University of Basel researchers successfully engineered and implanted autologous cartilage grafts using Chondro-Gide, a semi-permeable porcine type I and III collagen membrane scaffold (Fulco, 2014). Lan, who is defending her doctoral dissertation later this year, says the clinically approved Chondro-Gide scaffold was an obvious control when designing their study, but the 3D bioprinting protocol she had in mind sought to further improve surgical outcomes.
Dr. Adesida, who is a professor of surgery and advises Lan, is confident that 3D bioprinting nasal cartilage grafts will offer a competitive advantage by significantly streamlining what happens in the operating room. “With the scaffold-engineered cartilage, surgeons are limited by the supplier’s dimensions,” he says, pointing out a significant drawback with the rectangular Chondro-Gide scaffold strips that surgeons must cut and shape in the operating room.
Designing bespoke grafts

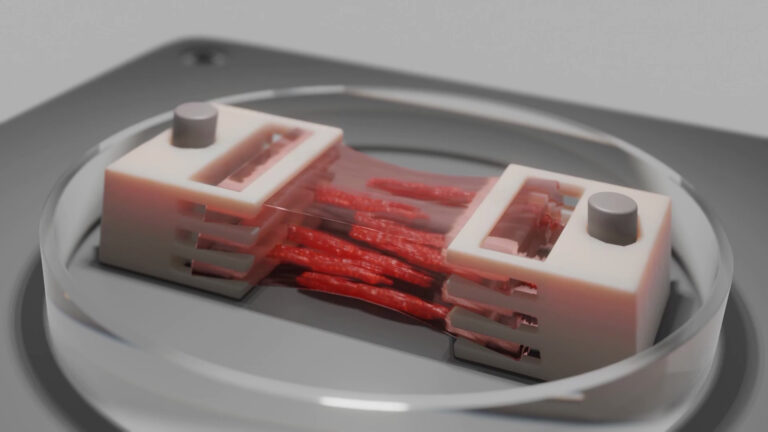
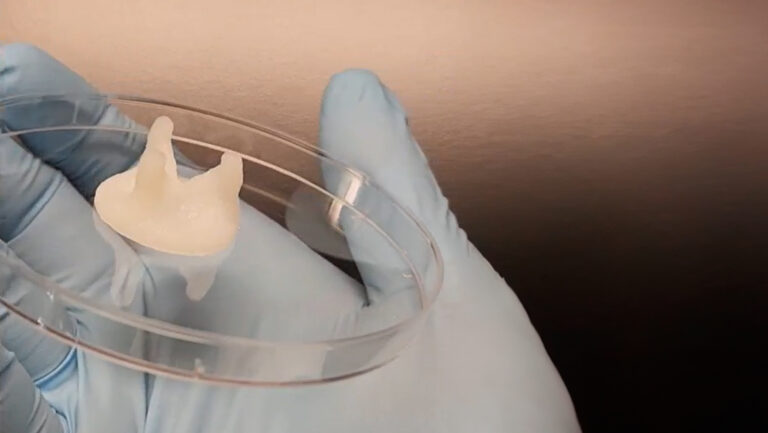
In the bioprinting strategy, however, CELLINK’s INKREDIBLE+ 3D bioprinter enabled the anatomical shaping of constructs based on computed tomography (CT) scans. When translated to the clinical setting, this protocol could help surgeons forgo hours of carving in the operating room. Dr. Adesida, whose lab focuses on developing autologous cell-based tissue-engineering strategies, envisions reconstructive surgeons easily placing the customized graft, suturing it in place, and sending the patient on their way.
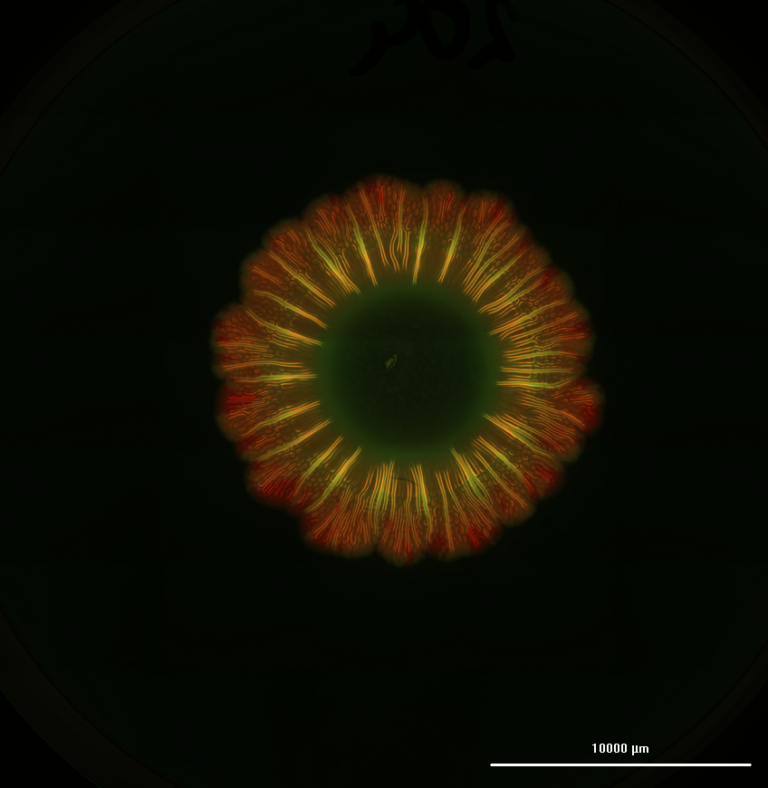
“Animals, humans, you name it,” quips Dr. Adesida. “Collagen is the favorite biomaterial of living organisms.” Fittingly, his lab opted to use Advanced BioMatrix’s LifeInk 200 type I collagen for this study. Lan notes that LifeInk 200 offers greater cell-biomaterial interactions and that by pairing the bovine-derived bioink with freeform reversible embedding of suspended hydrogels (FRESH) bioprinting, she was able to resolve the softness that can hamper the printability of animal-based hydrogels. Added bonus? The LifeInk bioprinted model showed more homogeneous extracellular matrix (ECM) distribution than the control Chondro-Gide scaffold-engineered grafts, which only developed cartilage matrix on the permeable side of the membrane.
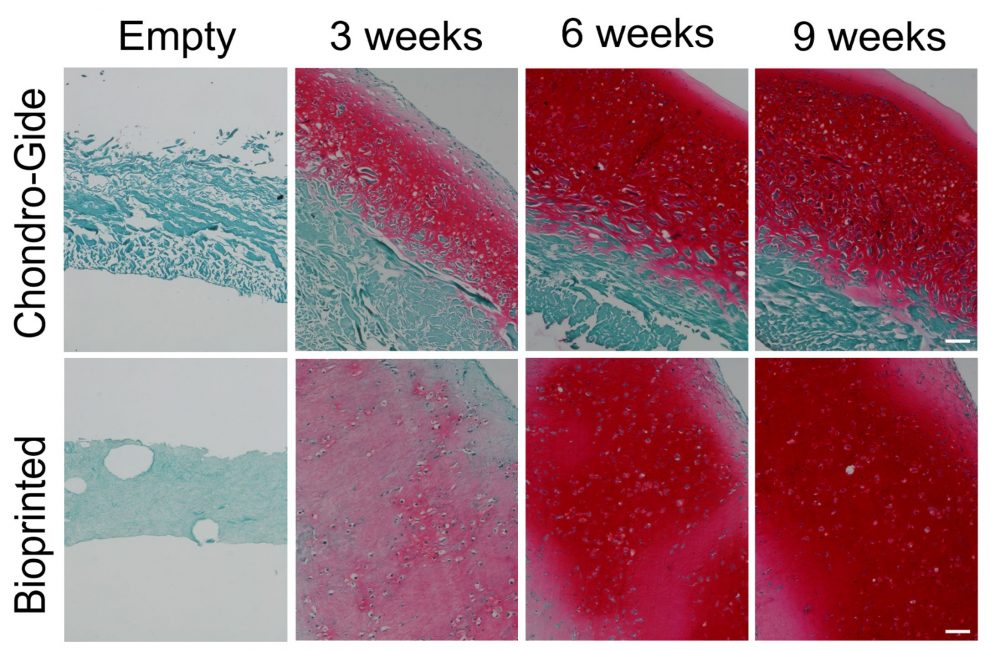
No further remodeling
With any reconstructive surgery, remodeling is a major concern. Weeks after implantation, patients could end up with warped noses, for example, as cells remodel the ECM during healing. Therefore, Dr. Adesida stresses the need to mature the 3D bioprinted cartilage in vitro for 7 to 9 weeks before implantation; at which point, the construct should withstand suturing, resist reshaping and avoid further remodeling. “With time, the bioprinted cartilage graft’s mechanical properties improved,” says Dr. Adesida of this promising outcome. Furthermore, past the 5-week observation period of the subcutaneously implanted graft in the murine model, it is unlikely that the 3D bioprinted cartilage would turn into bone, ensuring that patients could still breathe and flex their nose.
Next steps
According to Dr. Adesida, crucial next steps include making 3D bioprinting more financially attractive for surgeons and the healthcare system by reducing the time it takes to mature the 3D bioprinted cartilage in vitro. Right now, the Chondro-Gide model takes about 4 weeks to culture, as opposed to 7 to 9 weeks with the LifeInk model. Once they have narrowed that gap, the team is looking forward to clinical trials. “No one is using 3D bioprinted engineered nasal cartilage clinically in reconstruction for skin cancer patients,” notes the professor. With continued support from CELLINK and Advanced BioMatrix, the Adesida Lab hopes to be the first!
Learn more
Lan, et al. J Tissue Eng. 2022. DOI:10.1177/20417314221086368.
Lan, et al. FASEB J. 2021. DOI:10.1096/fj.202002081r.
Fulco, et al. The Lancet. 2014. DOI:10.1016/s0140-6736(14)60544-4.