A Colorectal Cancer 3D Bioprinting Platform for Disease Modeling
Tell us about your colorectal cancer research
Yordan Sbirkov
Colin McGuckin
Victoria Sarafian

“The BIO X’s flexibility, speed and reproducibility were instrumental in successfully printing our 3D colorectal cancer models,” says Dr. Sbirkov, who developed his protocol with this BIO X bioprinter in Dr. Sarafian’s lab.
Did you develop your own bioprinting protocol?
Yordan Sbirkov
Yes, and an interesting part was finding the right bioink. We used CELLINK’s RGD bioink because it’s functionalized with the short peptides that make cells feel more at home, communicate with each other, and cue each other, but also because it’s relatively clear. When we culture cells in a bioink, we need to monitor their progress. We take bioprints from different time points and examine them under standard fluorescent microscopes. If we cannot see the cells, we have to stop the experiment.
Colin McGuckin
Victoria Sarafian
We also used CELLINK’s BIO X™ to bioprint the 3D colorectal cancer models, which didn’t take very long. Then we used several techniques to assess the bioprinted tumors. Tissue staining was used to determine whether these were really the cancer cells we were looking for. Cell viability was assessed at different time points to ensure that our protocol was working well and that we would have the amount of cells needed for the next step. It was a long, tricky process, but we’re quite satisfied with the results we got with the protocol developed with Yordan.
What were some of the challenges unique to colorectal cancer research?
Yordan Sbirkov
Colin McGuckin
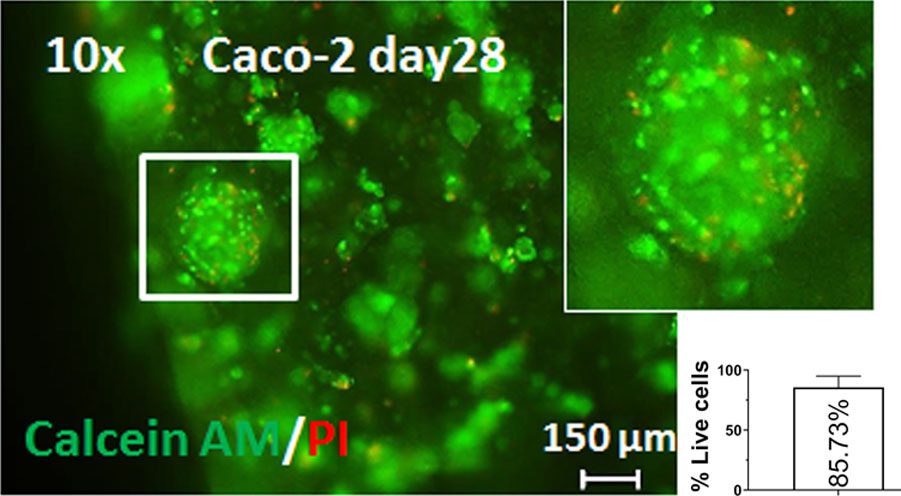
Green staining of live colorectal cancer cells reveals an 85.73% survival rate 28 days after bioprinting on the BIO X.




