Decoding Traumatic Brain Injuries with 3D Bioprinting
“While animal and 2D models have advanced our understanding of blood-brain barrier biology, 3D bioprinted models offer the ability to use human primary cells to bridge the gap to clinical translation.”
—Dr. Galpayage Dona
The majority of the 70 million traumatic brain injuries (TBI) suffered globally every year are nonfatal. “But the damage does not stop there,” cautions Kalpani N. Udeni Galpayage Dona, PhD, a postdoctoral fellow at the Servio H. Ramirez Laboratory of Neurovascular Research, Department of Pathology & Laboratory Medicine, Lewis Katz School of Medicine at Temple University in Philadelphia. “Even years after an initial brain injury, we see changes in the receptor expression, as well as altered ion channels, resulting in cell death and some neuronal dysfunction, which can lead to chronic or lifelong physical, cognitive and behavioral disorders, including mood swings, depression and anxiety.”

The blood-brain barrier
The highly selective permeability of blood vessels in the central nervous system, also known as the blood-brain barrier (BBB), determines which substances can enter or leave the cerebral blood flow to maintain homeostasis and protect the brain from blood-borne pathogens and toxins. Previous studies done with animal models or 2D cell culturing have suggested that the tight junctions between endothelial cells in the BBB could be damaged during a TBI. Dr. Galpayage Dona acknowledges these advances but argues that 2D cell culturing fails to capture the full complexity of the neurovascular unit that makes up the BBB. While endothelial cells do form the lumens through which blood flows and can fulfill the function of the BBB, the Florida Atlantic University graduate believes that endothelial interaction with adjacent cells, including neurons, pericytes and astrocytes, is required for regulation. Therefore, to properly study the effect of TBI on the BBB, it is necessary to develop a multicellular 3D model that better recapitulates the in vivo environment.
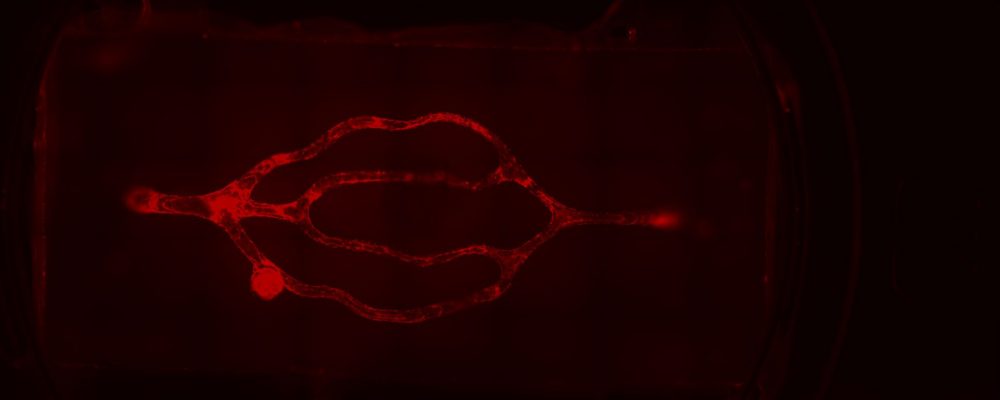
Light-based 3D bioprinting sheds new light
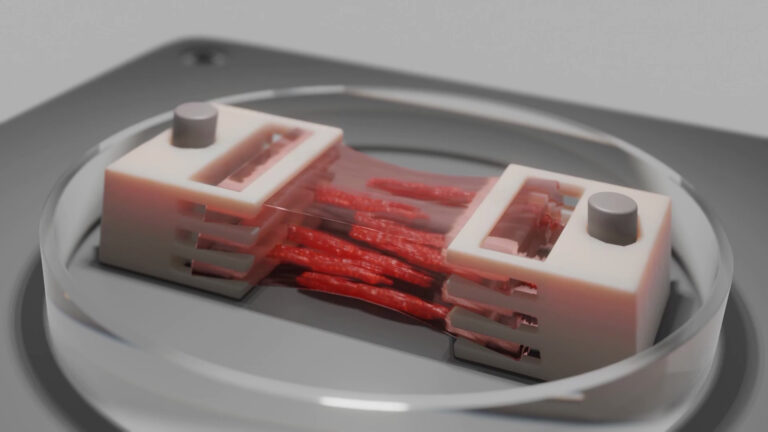
To recreate the intricacies of the brain’s 3D microvasculature and the microfluidic properties of in vivo vessels, Dr. Galpayage Dona turned to CELLINK’s light-based LUMEN X™ 3D bioprinter. The LUMEN X, the first DLP printer developed specifically for use with sensitive biomaterials and live cells, features an intuitive interface for uploading STL files of 3D CAD designs that are sliced into layers, which crosslink in as little as 10 seconds for unmatched speed. CELLINK’s light-based bioprinter also offers XY resolution down to 50 µm and Z precision down to 5 µm. Beyond the LUMEN X’s ease of use, speed and precision, Dr. Galpayage Dona says that the flexibility afforded by light-based bioprinting was instrumental as the bioprinted construct included a 3D scaffold of the brain’s microvessels, which ranged in diameter from 100 microns to 300 microns.
After some trial and error, Dr. Galpayage Dona found that to bioprint the 3D microfluidic scaffold, a combination of PEGDA PhotoInk™* and GelMA PhotoInk™* worked best. The resulting 3D cube of microvessels was then connected to a perfusion apparatus with two needles to perfuse the culture medium through, mimicking blood flowing through microvessels.
“I seeded the primary human brain microvascular endothelial cells onto the scaffold the next day because if we seeded the endothelial cells on the same day, the cells did not attach to the ECM,” says Dr. Galpayage Dona. “And I successfully cultured both fetal and adult brain microvascular endothelial cells in the scaffold and observed fully endothelialized vasculature after a few days.”
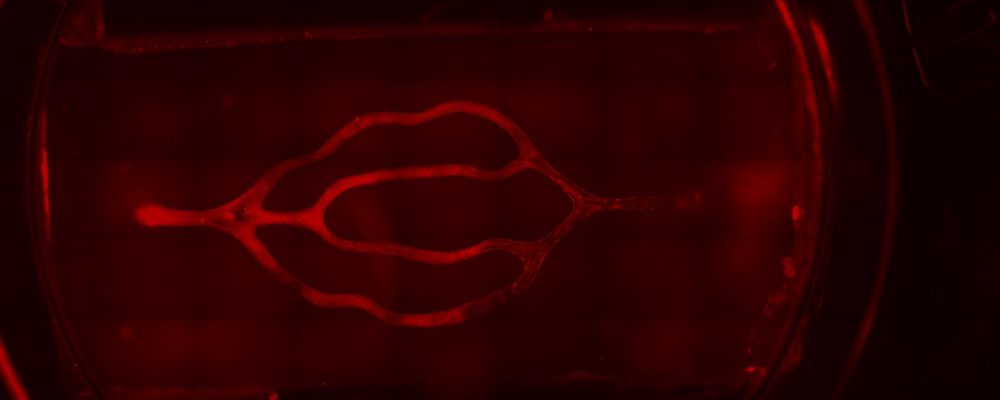
Next steps
While some have used everything from scratch tests to compression to supersonic shock waves to study TBI in vitro, a majority of researchers, including Dr. Galpayage Dona, choose the stretch model. Day 7 was the optimum day for the sake of confluence to use her stretching system to apply tensile strain to the fully endothelialized 3D model, but results did not show a significant difference between the permeability of the control group vs the TBI group once she perfused the 3D model with the drug dextran, which should be blocked by the BBB. Next steps will include “investigating how the severity of the TBI affects the dysfunction by trying different levels of strain and at different time points,” she says.
Another future step is to better understand how the TBI affects the neuronal circuitry. “We did not see the neural stem cells differentiating within our current PhotoInk combination inside the scaffold, so we are still investigating what combination of hydrogels will make this happen,” says Dr. Galpayage Dona, who differentiated neural stem cells on top of the scaffold on a glass slide, then stimulated them with light exposure to track the path of the neuron messaging. “Once we successfully differentiate the neural stem cells and astrocytes inside our 3D endothelialized scaffold, we can better reproduce neurovascular coupling and can see how these pathways are different before and after the TBI,” says the researcher, who intends to continue working with 3D bioprinted models.
Further reading
Galpayage Dona KNU, Hale JF, Salako T, et al. Frontiers in Physiology. 2021. DOI:10.3389/fphys.2021.715431.
* These biomaterials are no longer available and have been replaced with improved formulations