Boldly Going Where No Cell Has Gone Before
Bioprinting In Space
Despite pandemic-related setbacks, Joshua Chou, PhD, a senior lecturer at the University of Technology Sydney (UTS), is still working to send cancer tumors 3D bioprinted on the BIO X to the International Space Station (ISS). He hopes to study the mechanobiology of cancer. Dr. Chou’s lab at the School of Biomedical Engineering has already found that cancer cells die within 24 hours after exposure to microgravity. Yet he is quick to point out that they have no plans to send cancer patients to space for treatment.
“We want to expose our GBM-on-a-chip to the environment of space to see a different side of cancer cellular processes that we can exploit to our advantage,” explains Australia’s 2020 Space Researcher of the Year.

How did you get into cancer mechanobiology?
As part of my postdocs at Harvard Medical School in Cambridge and at Musashino University in Tokyo, I worked on the mechanical biology of bones and osteoporosis. With this other mechanically sensitive disease, I tried to identify the signaling pathways and how to block them. In essence, to manipulate cells and develop better therapeutics. By the time I was setting up my biomedical engineering lab back in Sydney, I chose to dig deeper into the mechanobiology of cancer. Years later, I am sending a cancer tumor to the ISS.
How are you using the BIO X to produce the tumor model you plan to launch?
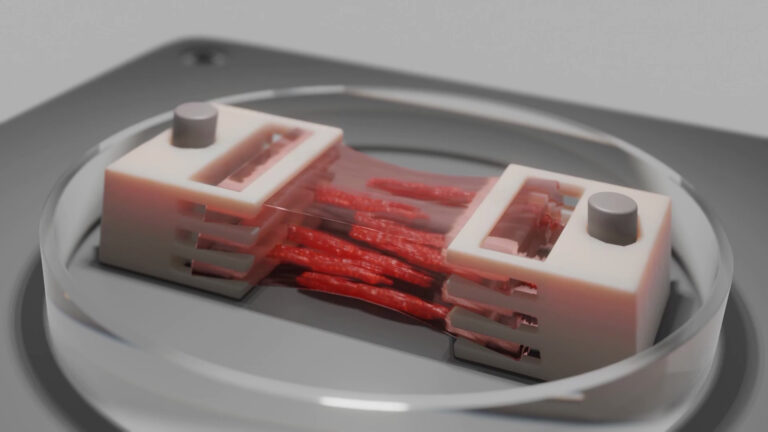
To mimic the glioblastoma (GBM) microenvironment, we developed a GBM-on-a-chip. At first, mixing cells manually in collagen gels was very labor intensive. We also couldn’t guarantee reproducibility from one experiment to the other. So, when CELLINK first came onto the market, I remember thinking, this is cool! I purchased a BIO X for my lab, and it has been a really rewarding experience.
The ability to automate the bioprinting of our GBM-on-a-chip with the BIO X is more efficient, more representative of the physiological environment and more reproducible. We 3D bioprint a glioblastoma tumor organoid into a central cavity surrounded by a mature vasculature that mimics the interactions between blood vessels and the glioblastoma.
We test which anticancer drugs go through the vascular channel, how the vascularization interacts with the glioblastoma and so on. Efficiently creating these glioblastoma spheroids was critical, and we didn’t spend a lot of time optimizing the workflow because the BIO X’s interface so straightforward.
Your launch has been delayed because of the pandemic. How have you been testing the effect of microgravity in the interim?
We used simulated microgravity devices rotating on the X and Y axes at a rate that essentially cancels out, or gets close to canceling out, the Z axis. We are recreating in the lab that zero gravity environment, which is 10-3 G. We can “launch” cells and see how they respond without going into real space.
How did the GBM-on-a-chip respond in microgravity?
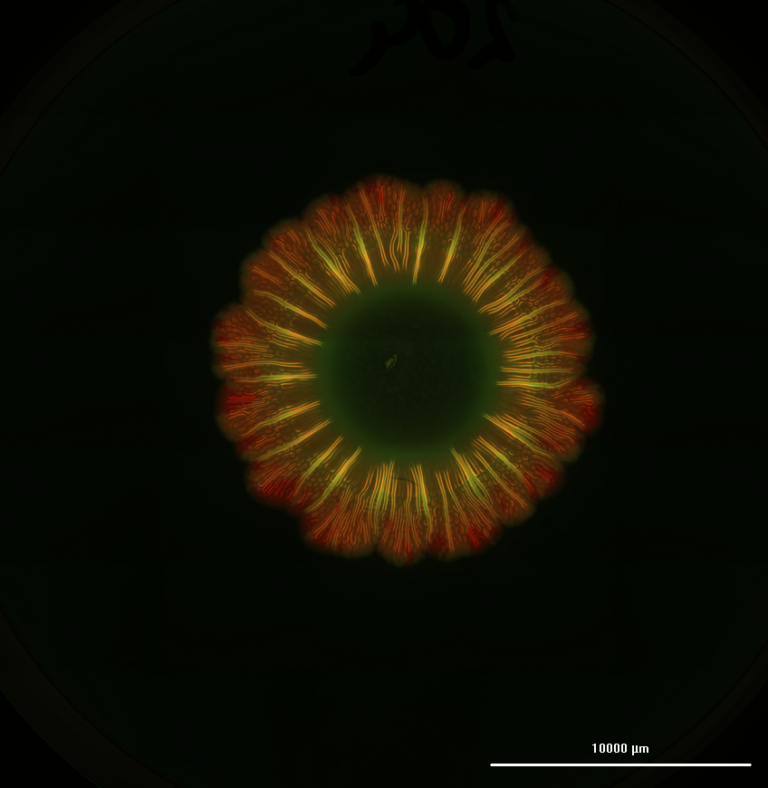
Microgravity is an alternative technology to decipher how cancers behave. When we started our research, it was to see if there was a fundamental functional mechanism that is preserved from one cancer to another.
Given that nothing on this planet has any genetic memory of being in the microgravity environment, we did expect a shock response. But in the simulated microgravity, we saw an aggressive response to mechanical unloading, or very low force, from all six of the cancer cells we looked at. A lot of the receptors or pathways got switched off or over sensitized, and the cells died within 24 hours.
Does this mean, we would take cancer patients to space for treatment?
That’s probably the No. 1 question I get! But no. The idea is not to send patients into space but to exploit the environment of space to better understand cancer.
For my postdoc, I looked into an antiosteoporosis drug that blocked off a signaling pathway in bone cells responsible for detecting mechanical loading. The drug tricked the cells into thinking they were mechanically active when they were not. Similarly with cancer, we want to identify a receptor or biomarker that detects sensory environments. Once identified, we want to block them and trick cancer cells into thinking they are in space when they’re still on Earth.
I think we’ll learn something even more unexpected from our launch because there will be a lot more factors at play. For example, we have found that some cells don’t care about microgravity but respond to radiation. Either way, our focus is to disrupt or disorientate cancer cells so we can increase the efficiency of currently available therapies.
What are some challenges to getting the GBM-on-a-chip to launch site?
With biological payloads going to the ISS, keeping cells dormant is one of the biggest problems. Cells are shipped at least 2 days before the launch. Then, it takes about 3 to 4 days from liftoff to reach the ISS. As cells proliferate, the modules will get pretty crowded. So, we are looking at safe hibernation at the cellular level during space travel.
How has your partnership with CELLINK affected your work?
Although the BIO X is an easy-to-use, plug-and-play device, the real value is in the CELLINK team’s support and expertise. As we started out, we needed to understand which gels would work for our application, and the team’s intimate knowledge of their bioink portfolio was invaluable.
We’ve worked well with the CELLINK team for the past 4 years. My students and I feel comfortable reaching out whenever we need help troubleshooting. In fact, I have been talking about partnering and looking into testing different bioinks from CELLINK under microgravity. The work will be interesting in itself and will hopefully add value to other bioengineers looking into space research.