More Efficient Drug Screening with 3D Bioprinting
Taking a drug to market is a competitive, costly and challenging process involving preclinical laboratory and animal testing before the even more time-consuming and expensive four phases of human clinical trials, which can take as many as 7 to 15 years at price tags as high as $5.5 billion. Even if 10 viable drug compounds are identified for human trials, only 1 out of 9 will actually make it to market. Given this high attrition rate, can bioprinting save valuable time and resources by better identifying viable compounds in order to move only the most promising drugs to clinical trials?
Limitations of animal models for drug screening
During the initial stages of drug discovery, often referred to as preclinical trials, new chemical entities (NCEs) are monitored to determine the life cycle of the compounds inside and outside of the targeted system (pharmacokinetics) and their chemical reactions (metabolism). Because of the ethical issues surrounding human trials and their high costs, a significant number of these early tests are performed on animals.
While the transition from preclinical animal testing to clinical human trials has improved thanks to better research tools and the rise of artificial intelligence in target identification, there is still a real need for improved preclinical screening because animal testing often fails to recapitulate the complexity of the human metabolism, leading to false positives and negatives that do not accurately reflect the toxicity of drugs to human systems.
3D cell cultures are more relevant
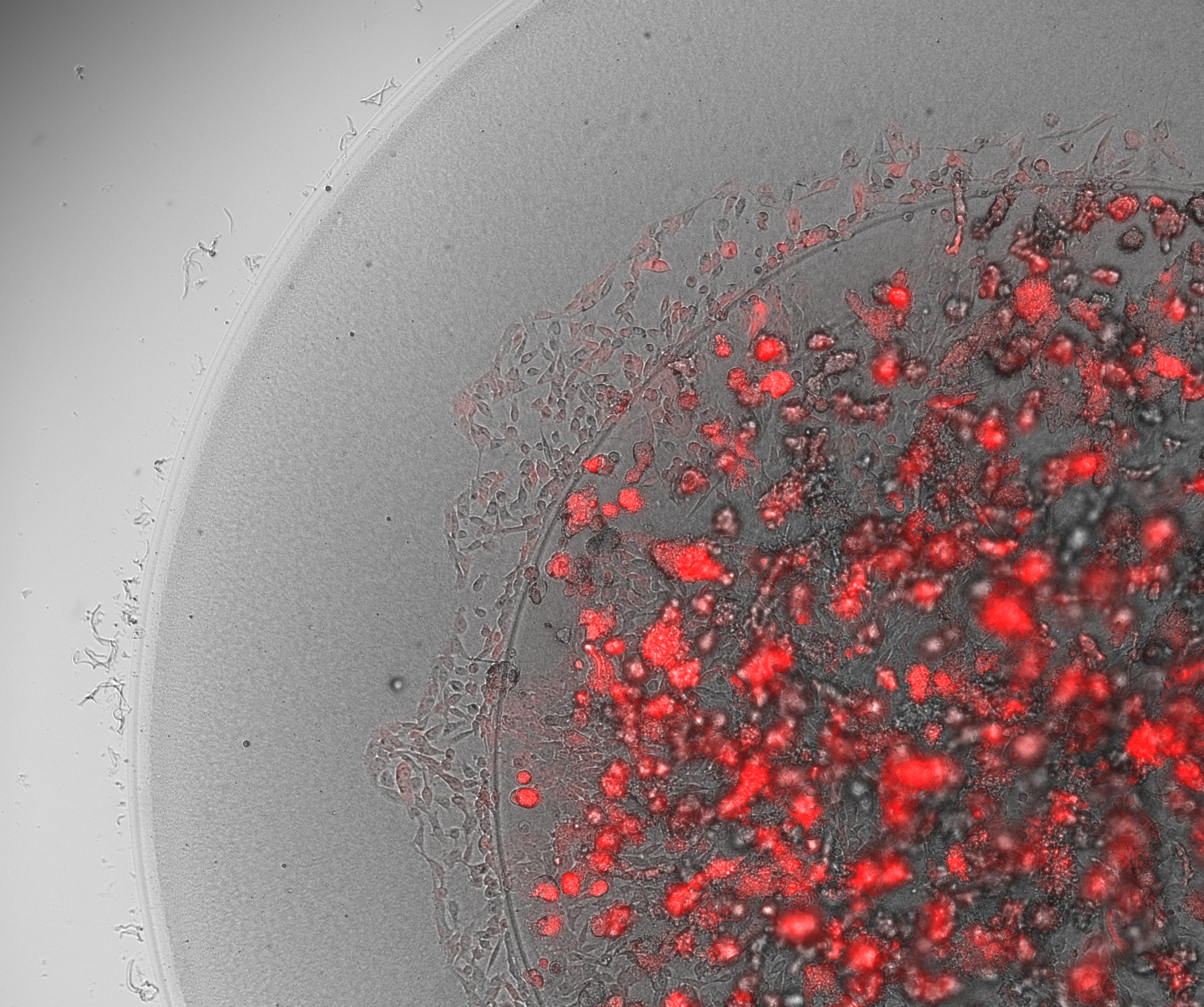
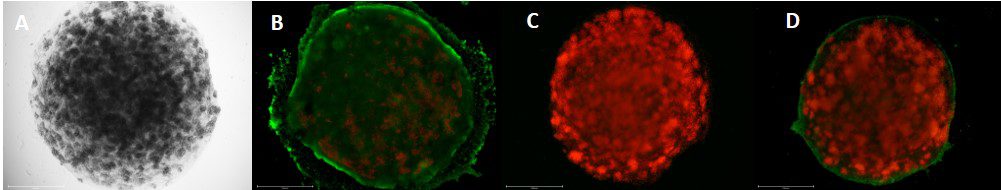
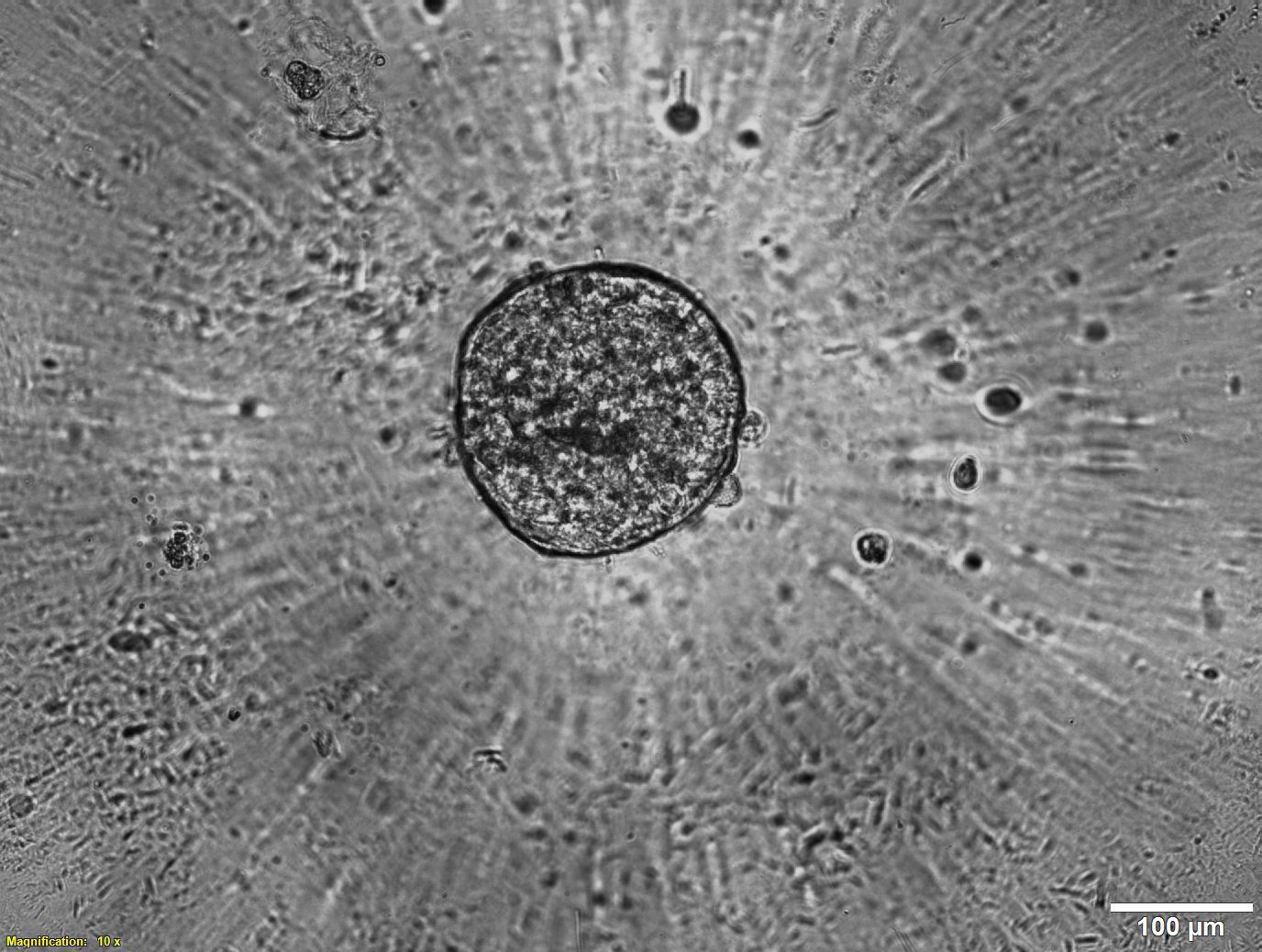
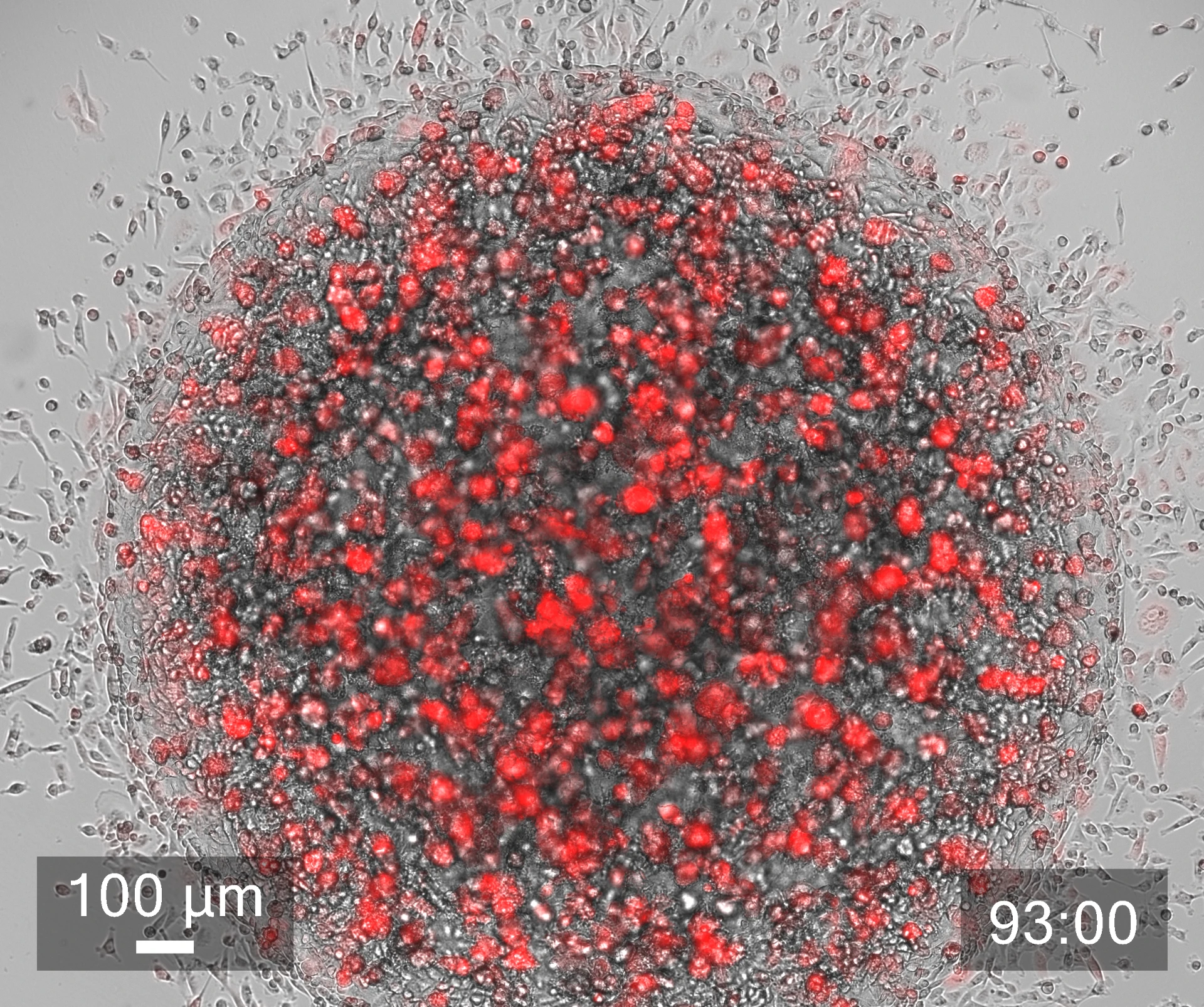
Given the limitations of animal models, it is no wonder that scientists have turned to human organ models. But although human cells have long been cultured in 2D, in recent years, a paradigm shift has led more and more scientists to recognize the importance of working with human cells in the 3D environments afforded by bioprinting in order to produce more physiologically relevant models. Combining the automation of cell culturing in 3D bioprinting with carefully tailored biomaterials, known as bioinks, has made it possible to grow, feed and maintain human organ models in larger quantities and in a lot less time, reducing time and labor spent on these tasks. Laboratory robotics can also now pick and place cell culture reagents or other NCEs and liquid samples in high numbers, enabling higher throughput screening and running a variety of other laboratory tasks more efficiently.
Bioinks better mimic ECM
Bioinks are another powerful tool that help researchers advance their drug discovery research. Tissue–specific bioinks improve cell adhesion and differentiation, helping with the formation of human organoids. Proteins and other biological factors can also be added to more accurately recreate extracellular matrices (ECM) and better simulate in vivo microenvironments. Furthermore, with multiple methods of crosslinking (chemical, light, thermal), the stiffness of constructs can be modulated to better serve specific cell types, like cartilage or bone tissue.
Learn more
Bioprinting’s more relevant human organ models can save the drug industry time and money by more efficiently identifying viable compounds in the initial stages of drug development. Conceivably only the most promising compounds would move on to costly human clinical trials. The technology’s growing influence means that scientists continue to validate more and more applications. Watch our webinar on 3D bioprinting for COVID-19 studies.