Collagen models of drug-induced liver injury
Collagen, which contributes to tissue structure, cell-cell interactions and cellular reorganization, is widely used in tissue engineering studies. Its abundant binding sites, easy gelation and attractive degradation properties make it a versatile bioink for most tissue models, including the liver.
In a recent study, a team of CELLINK scientists bioprinted collagen ‘mini livers’ to evaluate drug-induced liver injury (DILI), a leading cause of acute liver failure, hepatitis and fibrosis.1 Collagen I was chosen for this study because of its high presence in hepatic tissue and its favorable properties in 3D cell culture.3 Additionally, collagen’s ability to be uniformly crosslinked and droplet-printed is unmatched by other biomaterials. The mini liver models were made up of multiple hepatic and non-hepatic cell types, including HepG2, LX2 and HUVECs, in order to mimic liver functions such as albumin secretion, lipid accumulation and alanine aminotransferase activity, a known marker of liver injury. Each model consisted of one cell-laden collagen I droplet, inside another cell-laden collagen I droplet. Droplet encapsulation was employed because it produced a highly functional liver model with relevant nutrient and oxygen gradients. Encapsulation also provided a unique opportunity to study cell communication, control cellular arrangement and allow for different stiffnesses between layers of tissue.
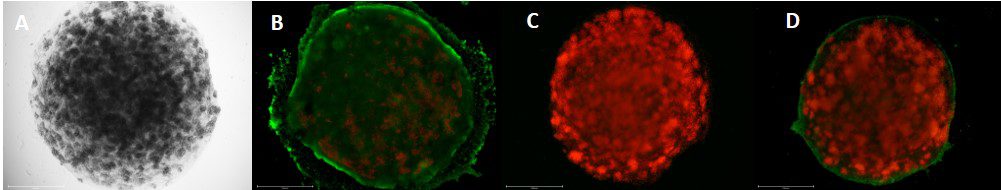
An assessment of hepatoxicity showed that the mini liver models responded adversely to high doses of acetaminophen and flutamide, drugs that have been linked to DILI. These results suggest that mini liver models can be implemented for drug screening as an accurate predictor of drug toxicity and as a high-throughput alternative to animal studies, 2D cell culture and organ-on-a-chip models.
Collagen’s versatility makes it the bioink of choice in many other studies. For instance, collagen’s degradation patterns or rheological properties could be of interest when studying liver injury and inflammation. The latter are associated with tenderness and enlargement in the liver and could warrant future studies that model different stages of fibrosis and evaluate cell migration in tissues of varying stiffnesses.
Bioprinting with collagen
Despite its versatility, collagen is a notoriously temperamental biomaterial that is sensitive to changes in temperature, pressure and rheology. Printability can often seem challenging; but rest assured, there are several ways to increase the printability of collagen. Here are just a few:
- Combine low-viscosity collagen with a thickener or another biomaterial such as alginate
- Bioprint low-viscosity collagen in FRESH LifeSupport or a similar microparticle slurry
- Consider ColMA, a photocrosslinkable collagen, for enhanced mechanical stiffness and printability
- Use a Temperature-controlled Printhead to bioprint or droplet-print collagen
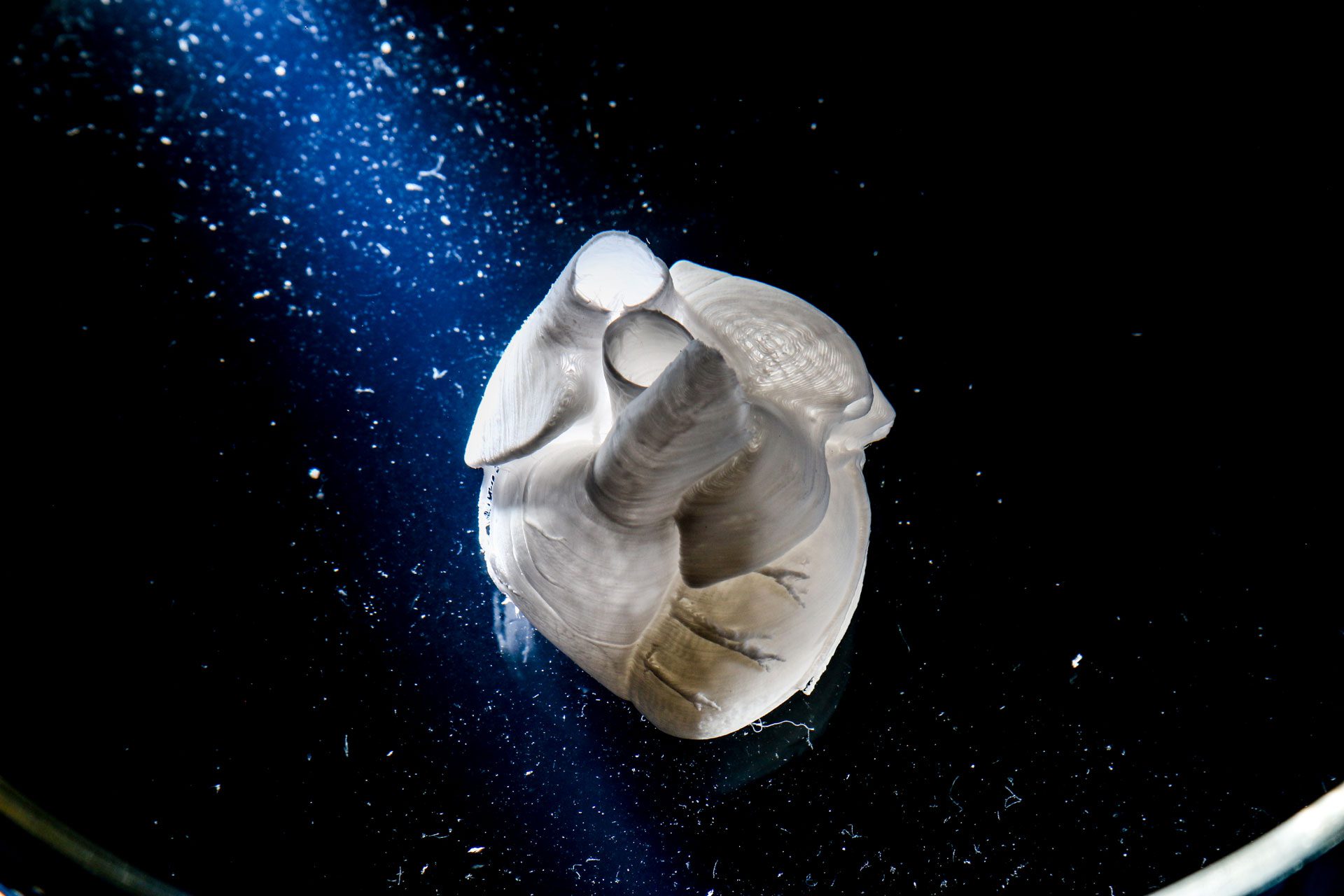
As one of the most prominent proteins in the body, collagen plays a major role in tissue engineering research. From rebuilding parts of the human heart2 to targeting tumors with cytotoxic T cells4 to discovering anti-aging mechanisms, the potential research applications are numerous. So, indulge us, what will you print with collagen?
References
- David, S., and Hamilton, J. P. Drug-induced Liver Injury. S. Gastroenterology & Hepatology Review. 2010; 6: 73–80.
- Lee, A., Hudson, A.R., Shiwarski, D.J., et al. 3D bioprinting of collagen to rebuild components of the human heart. 2019; 365(6452): 482–487.
- Martinez-Hernandez, A., and Amenta, P. S. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Archiv. A pathological anatomy and histopathology. 1993; 423(1): 1–11.
- Pruitt, H. C., Lewis, D., Ciccaglione, M., et al. Collagen fiber structure guides 3D motility of cytotoxic T lymphocytes. Matrix Biology: Journal of the International Society for Matrix Biology. 2020; 85-86: 147–159.