Droplet-in-Droplet Models
For Biomedical Applications
Droplet-in-Droplet Models, briefly explained
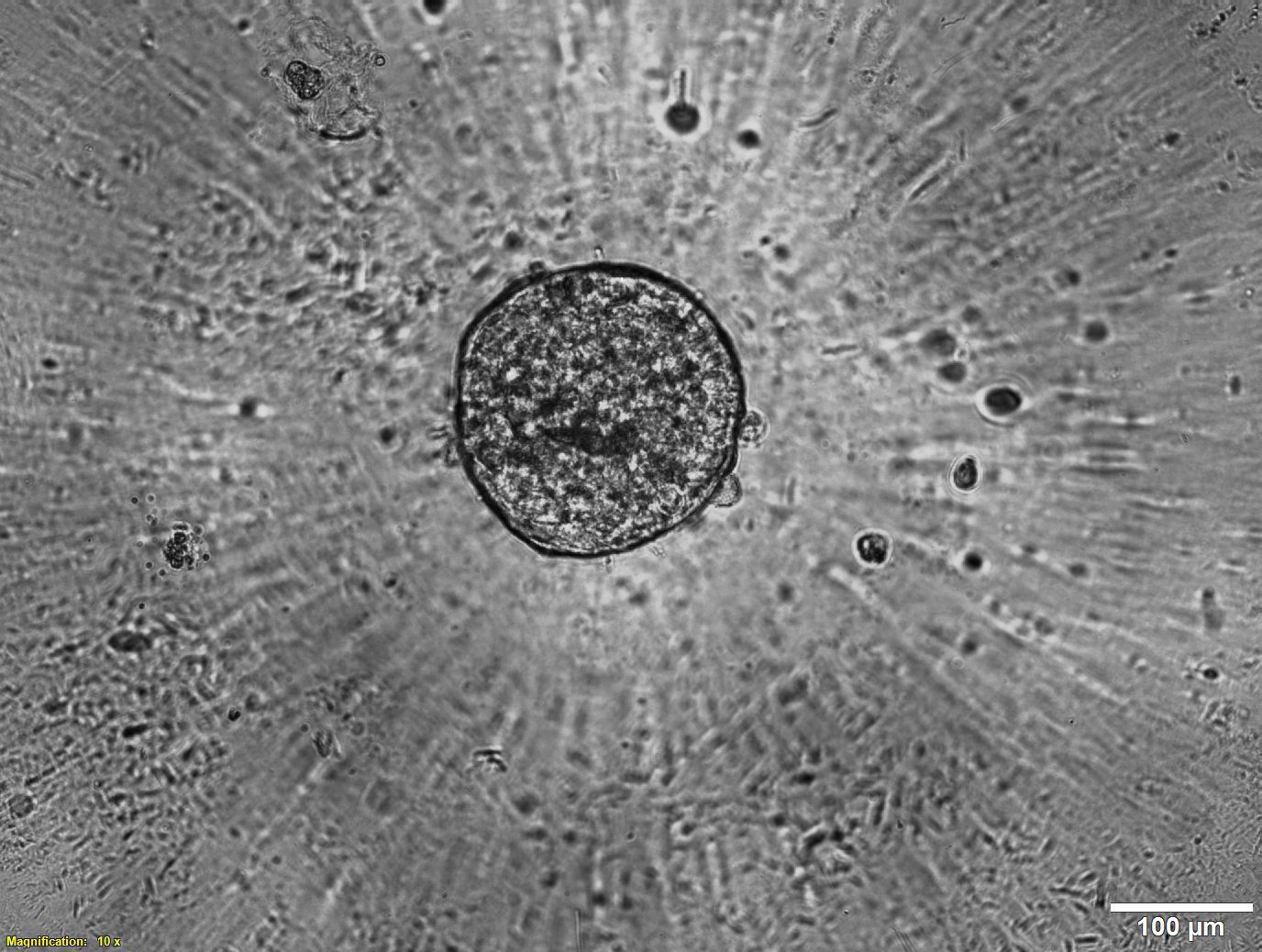
Droplet-in-droplet (DiD) printing is a biodispensing technique that involves encapsulating smaller droplets of hydrogels within larger droplets of another hydrogel. This technique creates droplet-in-droplet structures that act as miniaturized multi-material, multi-layer models. These models can be used for 3D cell culture to better mimic the microenvironments found in natural tissues.
By precisely controlling the composition of the cell-laden hydrogel (or bioink), droplet-in-droplet models can enhance cellular interactions and enable more accurate studies of tissue and cell behaviors.
How does droplet-in-droplet printing work?
One of the most important qualities for printing droplet-in-droplet structures is precision. To get a reliable, consistent, and reproducible result, you need to trust that you can reproducibly extrude droplets of the right size in the right area in space, ensuring full encapsulation of the inner droplets.
We have launched BIO ONE, a biodispenser which utilizes a mechanical syringe-based mechanism to print structures. The BIO ONE is the perfect system for printing droplets with minimal variation in the volume size. We built out both a Droplet-in-Droplet protocol as well as a Technical Note to showcase how you can print these models with the BIO ONE in up-to 48-well plates.
Droplet-in-Droplet Workflow
- Prepare the protocol in the software. We recommend a minimum volume of 5 µL for the inner droplet and 30 µL for the outer droplet. The outer droplet should be set-up to print above the inner droplet through a z-offset.
- Prepare the two bioinks and load them into 3 mL syringes.
- Load the first bioink into the printhead and attach a 20-22G nozzle. Calibrate the printer and prime the bioink.
- Print the inner droplets. Once printed, crosslink the droplets according to your protocol.
- Repeat step 3 for the second bioink but with a 18-20G nozzle, and print the outer droplet.
- After crosslinking the outer droplets, add desired cell medium and incubate the constructs according to your protocol.
Download Technical Note
Using DiD models for cancer invasion assays
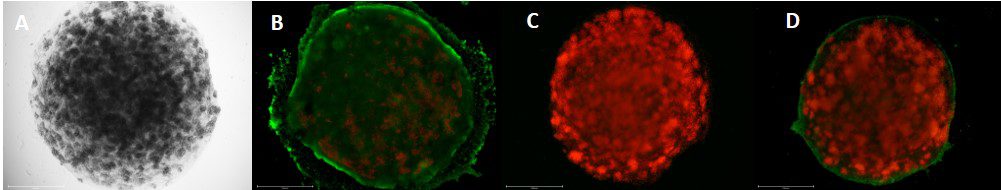
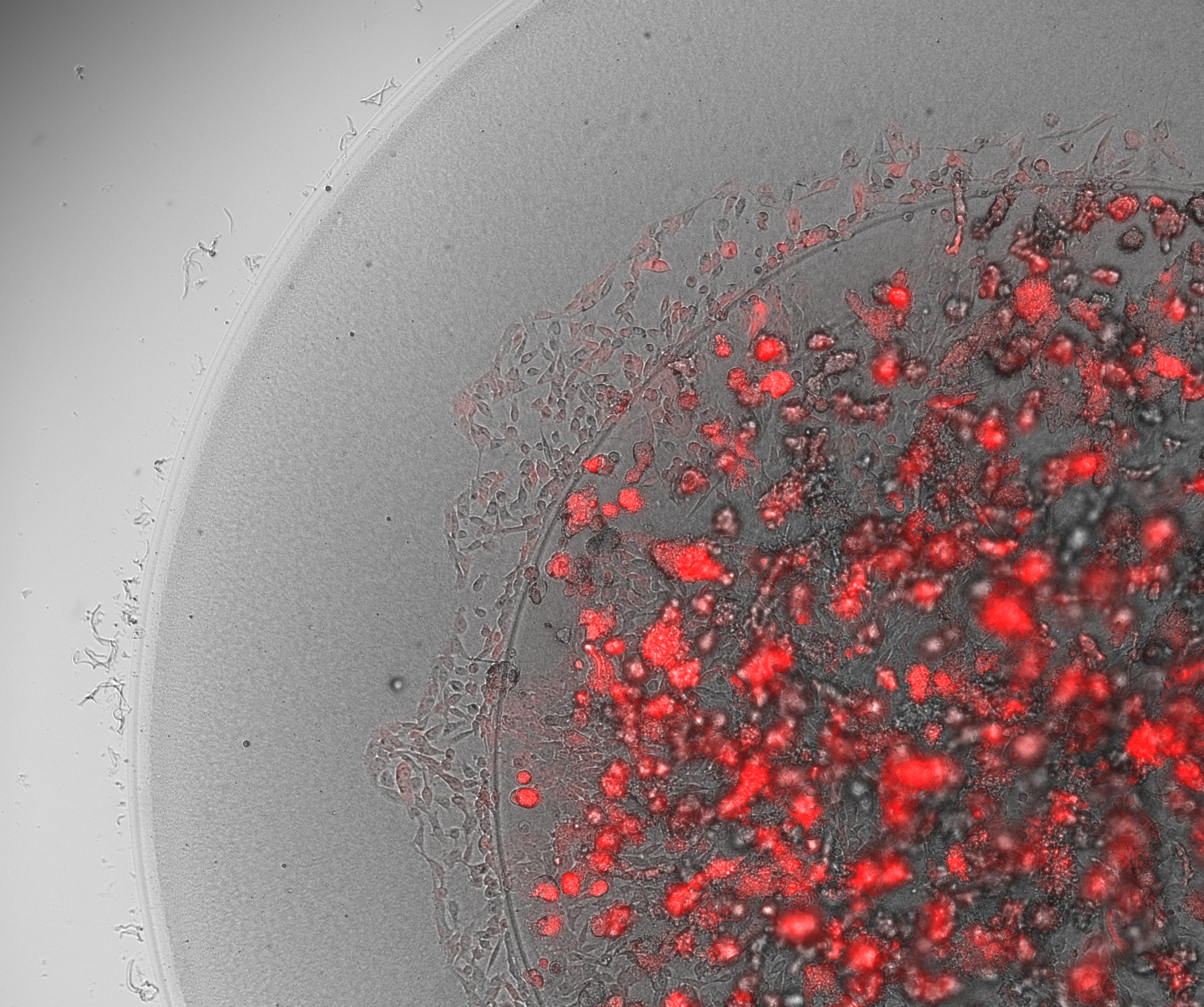
To illustrate the benefits of encasing droplets within droplets, we conducted an in-house experiment with cancer cells to study the migration of cancer cells.
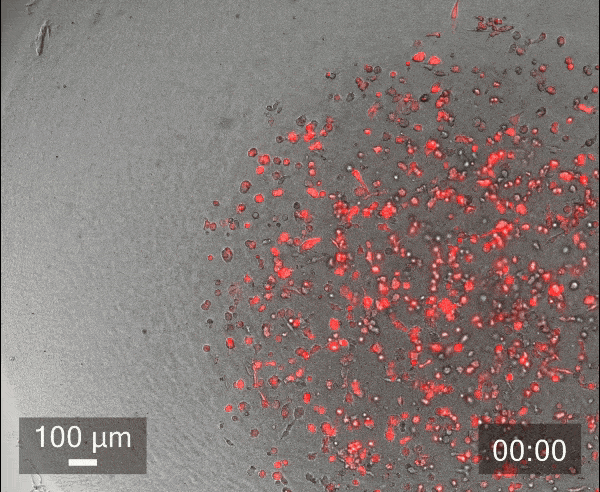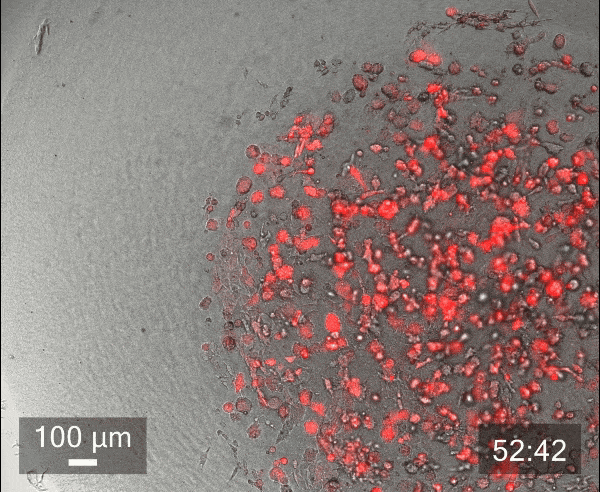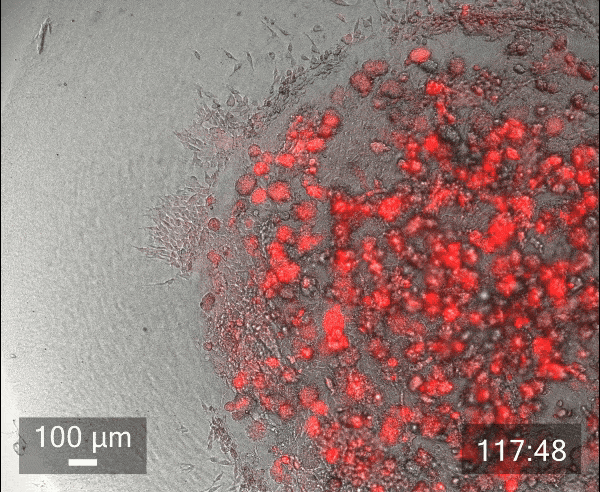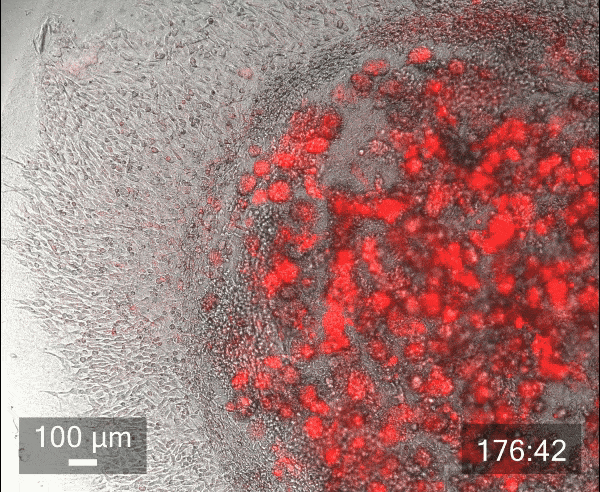
In this study, we printed inner droplets at 1 µL and outer droplets at 5 µL, utilizing TeloCol-10 at two different concentrations. The inner droplets at 6 mg/mL were laden with breast cancer cells (MDA-MB-231 mCherry), and the outer droplets (at 4 mg/mL) were cell-free. We repeated this experiment with Matrigel at the same concentrations.
These TeloCol-10 and Matrigel models were tracked with the Cellcyte 1 from Discover Echo every 3 hours for 9 days.
We could observe that the cells migrated from inner droplet to the cell-free outer droplet for both the TeloCol-10 and Matrigel models after 3 days, followed by a massive cell migration on subsequent days. We also saw the formation of spheroid clusters in the inner droplets.
Overall, we tracked the migrating cells in TeloCol-10 for the entire 9 days, while we could only track the Matrigel models for 7 days.
Cancer cell migration assays like these can help researchers understand metastasis, develop anti-cancer drugs, and study cellular mechanisms. These could also be used to analyze the migratory properties of patient-derived cells, which can inform treatment strategies tailored to the individual.
Other Applications
Co-Culture
Droplet-in-droplet models are a robust platform for co-culture as they maintain spatial organization while promoting interaction between different cell types.
Essentially, DiD models can create two distinct compartments. The inner droplet encapsulates one cell type, and the outer droplet another.
This mimics the complexity of the body’s tissue environments and can be used to study cell-cell interactions, paracrine signaling, and tissue-specific processes such as tumor-stroma dynamics, angiogenesis, and immune responses in a controlled microenvironment.
Immuno-assays
Droplet-in-droplet models offer a versatile platform for immuno-assays, enabling high-throughput and precise analysis of immune responses.
By encapsulating immune cells within droplets, these models would help detect the cellular response to the cytokines and antibodies produced by the cells. The small scale of the models allows rapid screening of immune activity, supporting with applications in drug testing, biomarker discovery, and vaccine development.
Miniaturized organ models (e.g. liver islets)
Droplet-in-droplet systems are highly effective for creating miniaturized organ models, enabling the study of organ-specific functions within a controlled, reproducible environment.
The inner hydrogel droplets, containing various cell types, can replicate the complex interactions and architecture of tissues, while the outer hydrogel droplets provide additional support and a controlled microenvironment. For example, liver, kidney, or pancreatic cells are arranged in the inner droplets, which mimic their native microenvironments, allowing researchers to observe processes such as drug metabolism, filtration, or insulin secretion.
This method allows for the study of organ functions at a smaller scale, offering enhanced sensitivity and precision. It is particularly valuable for applications in drug testing, disease modeling, and tissue engineering, providing a realistic alternative to in vivo conditions while enabling scalable, high-throughput experiments.
Conclusion
Droplet-in-droplet printing is a promising, powerful tool for biomedical research, offering precise control over 3D cell culture environments. By encapsulating inner, cell-laden, hydrogel droplets within larger hydrogel droplets, researchers can create biomimetic models that mimic the complexity of natural tissues. This technique enables the study of a wide range of biological processes, including cell migration, immune responses, and organ function.
With the BIO ONE, researchers can easily and reliably generate droplet-in-droplet structures, opening new possibilities for drug discovery, tissue engineering, and personalized medicine.