How can bioprinting be used for cancer research?
Cancer is a large group of diseases characterized by the abnormal growth of cells. It is the second leading cause of death globally, accounting for an estimated 9.6 million deaths, or 1 in 6 deaths, in 2018.
What is cancer?
Cancer can start anywhere in the body and can spread to many other parts. In cancer, healthy cells undergo DNA changes which cause them to divide uncontrollably. This division results in an overcrowding of cells that blocks essential functions and results in the growth of tumors. If a tumor is cancerous, it is defined as malignant and has the ability to invade neighboring tissues and spread throughout the body, wreaking havoc on bodily functions. Cancerous cells also have the uncanny ability to avoid our own immune system by ignoring killing signals and influencing other cells in the area to form networks of oxygen supply and waste removal that promote the growth of more cancerous cells.
While many patients suffer, various cancers continue to be a challenge for the doctors to diagnose and treat. Significant strides have been made over the past decade in treating cancer and developing diagnostic tools, but there is much to improve. This is especially true when it comes to the development of effective anticancer drugs. Traditionally, scientists within the pharmaceutical industry have relied on 2D cell culture to test cancer medications. While 2D models have guided much of our understanding to date and have played an important part in getting us to where we are, they fail to accurately recapitulate cell interactions that take place in cancerous tissues.
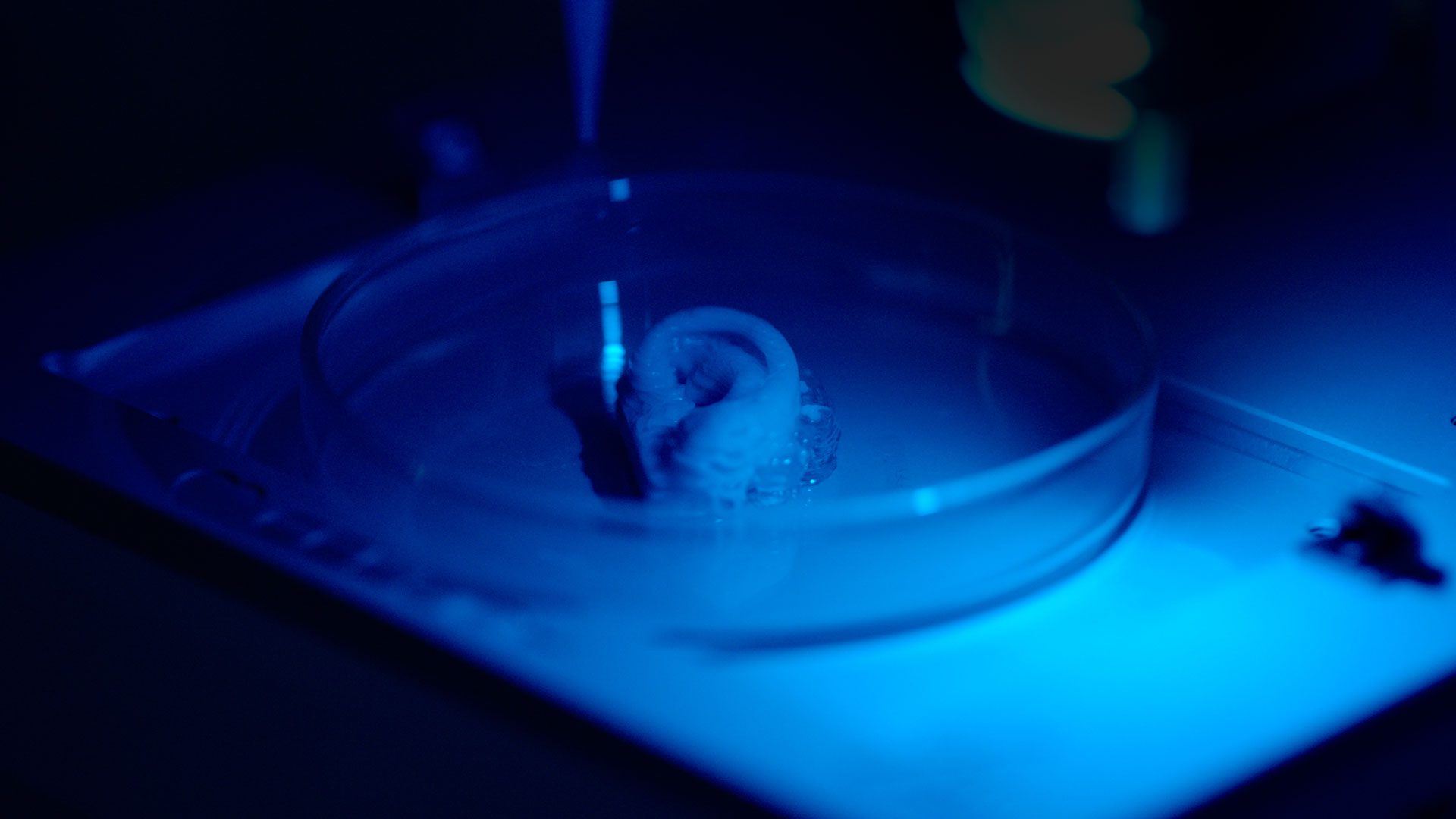
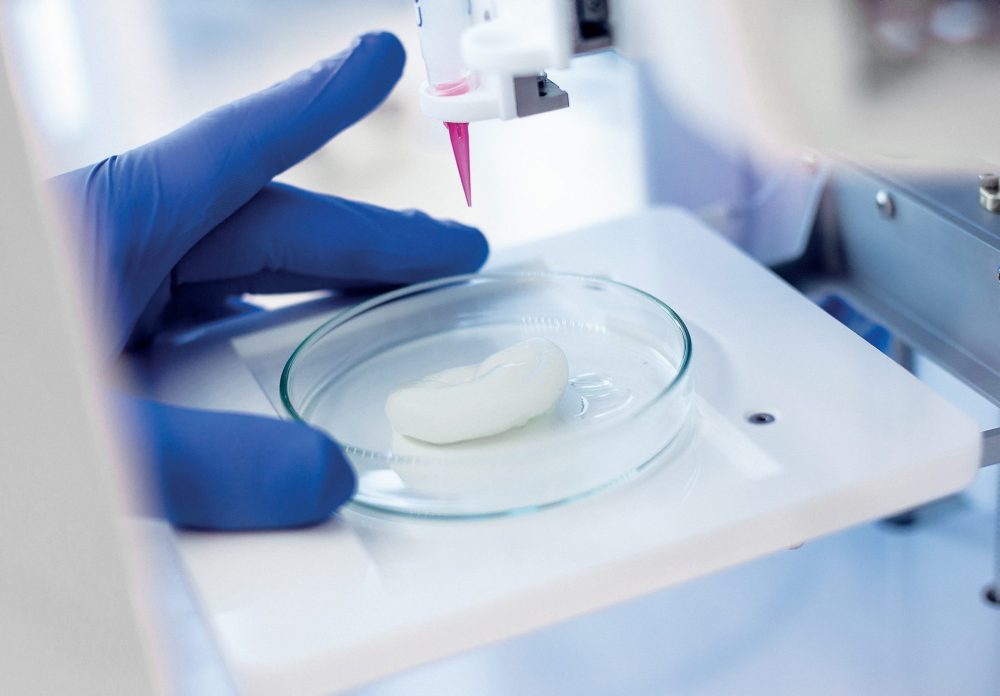
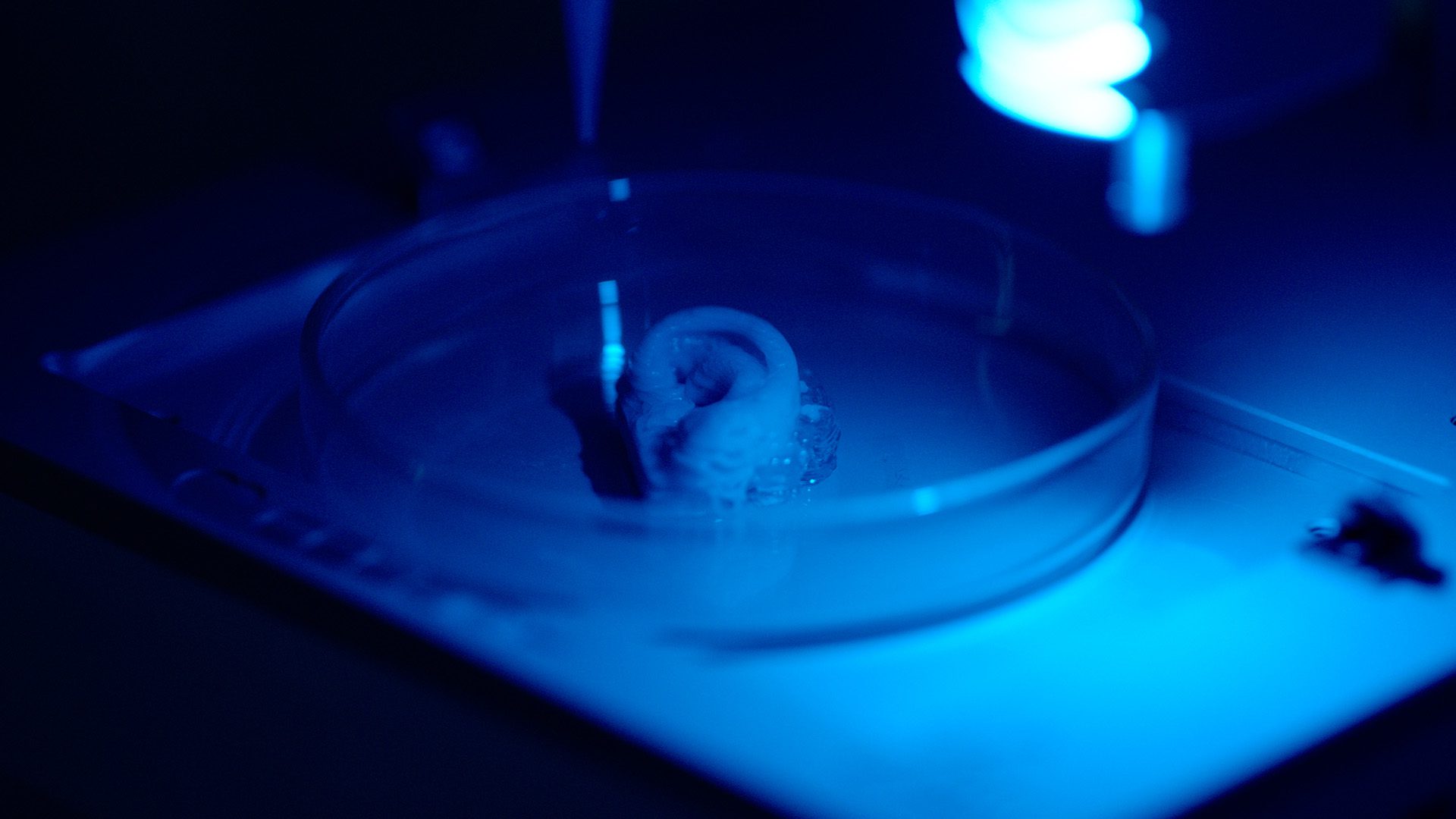
On the other hand, 3D bioprinting has shown exceptional promise and potential in helping us move forward from planar cell culture to develop in vitro models that are more physiologically relevant. More specifically, through automation, precision and the clever use of biomaterials, bioprinting provides a method to better mimic the complex tumor microenvironment in a repeatable and high-throughput manner.
While many patients suffer, various cancers continue to be a challenge for the doctors to diagnose and treat. Significant strides have been made over the past decade in treating cancer and developing diagnostic tools, but there is much to improve. This is especially true when it comes to the development of effective anticancer drugs. Traditionally, scientists within the pharmaceutical industry have relied on 2D cell culture to test cancer medications. While 2D models have guided much of our understanding to date and have played an important part in getting us to where we are, they fail to accurately recapitulate cell interactions that take place in cancerous tissues.
On the other hand, 3D bioprinting has shown exceptional promise and potential in helping us move forward from planar cell culture to develop in vitro models that are more physiologically relevant. More specifically, through automation, precision and the clever use of biomaterials, bioprinting provides a method to better mimic the complex tumor microenvironment in a repeatable and high-throughput manner.
In one of our webinars, Dr. Yoshie discussed the issues researchers must consider when exploring 3D bioprinting in the world of cancer research. Everything from the bioinks, cell types and structural designs to the printing conditions can influence biochemical interactions, how cells interact with each other and the overall accuracy of a model. Dr. Yoshie also presented specific fields within bioprinting that are currently being explored such as cancer microenvironment, cancer metastasis, cancer angiogenesis, drug screening and many more.
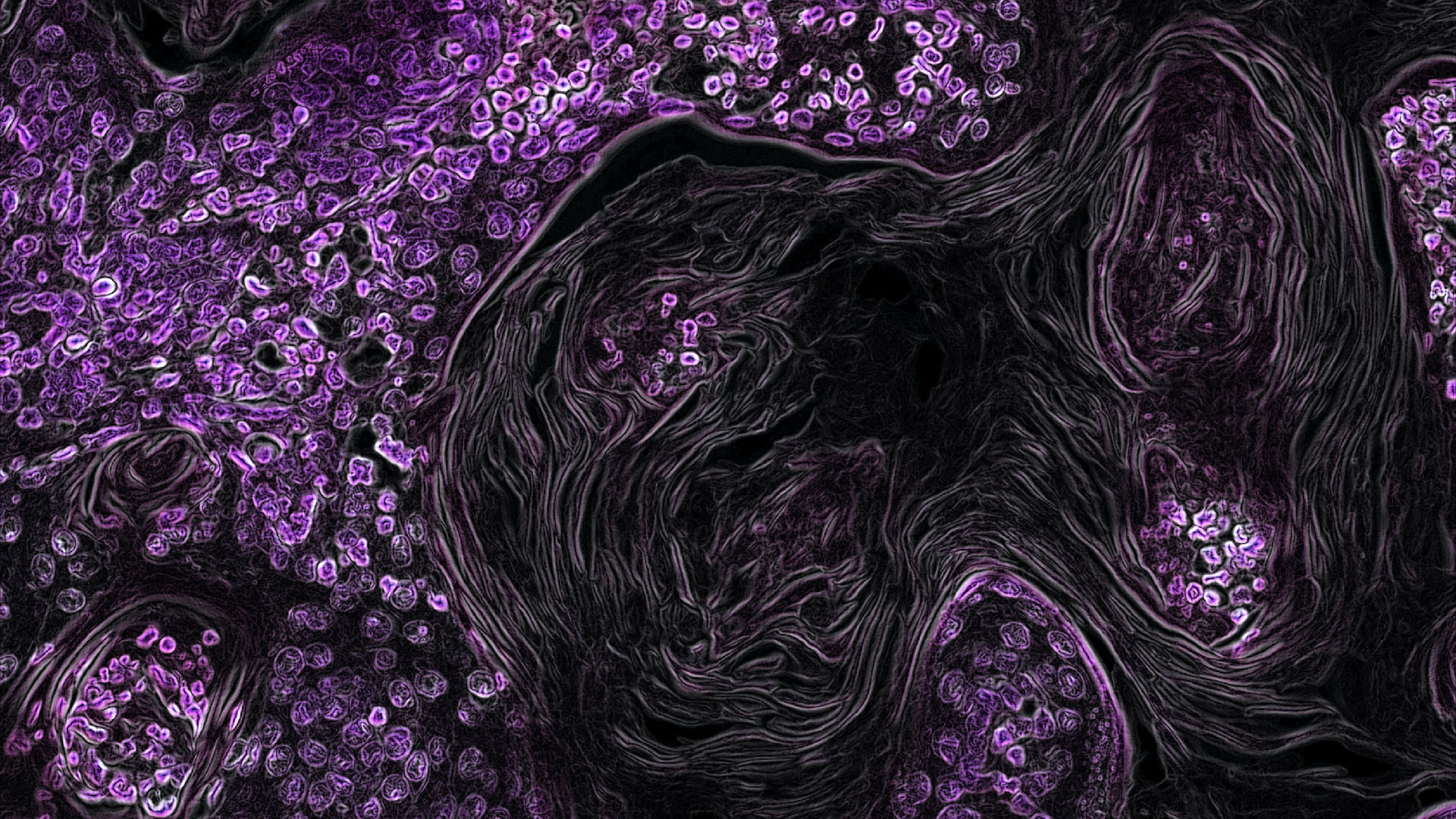
Dr. Yoshie outlined an interesting project that a current CELLINK customer was undertaking on cancer spheroids, in which they developed a nonsmall lung cancer model. The researchers co-cultured patient-derived tumor cells and cancer associated fibroblast (CAF) cells into their bioink and used the INKREDIBLE for their printing procedure. They investigated the printability, cell viability, cellular crosstalk and mechanical properties and observed that spheroid formation increased over time to produce in vitro tumor co-culture spheroids that were more physiologically relevant cancer models.
In addition to this, CELLINK scientists recently carried out an experiment to develop a syngeneic tumor model. They developed a murine lung cancer model using the BIO X and evaluated T cell killing efficiency. They were able to demonstrate that by running T cell cytotoxicity assays on 3D bioprinted cancer models, researchers can screen checkpoint inhibitors in a more efficient and translational manner. You can learn more about their work below or watch Dr. Yoshie’s insightful webinar.
Dr. Yoshie outlined an interesting project that a current CELLINK customer was undertaking on cancer spheroids, in which they developed a nonsmall lung cancer model. The researchers co-cultured patient-derived tumor cells and cancer associated fibroblast (CAF) cells into their bioink and used the INKREDIBLE for their printing procedure. They investigated the printability, cell viability, cellular crosstalk and mechanical properties and observed that spheroid formation increased over time to produce in vitro tumor co-culture spheroids that were more physiologically relevant cancer models.