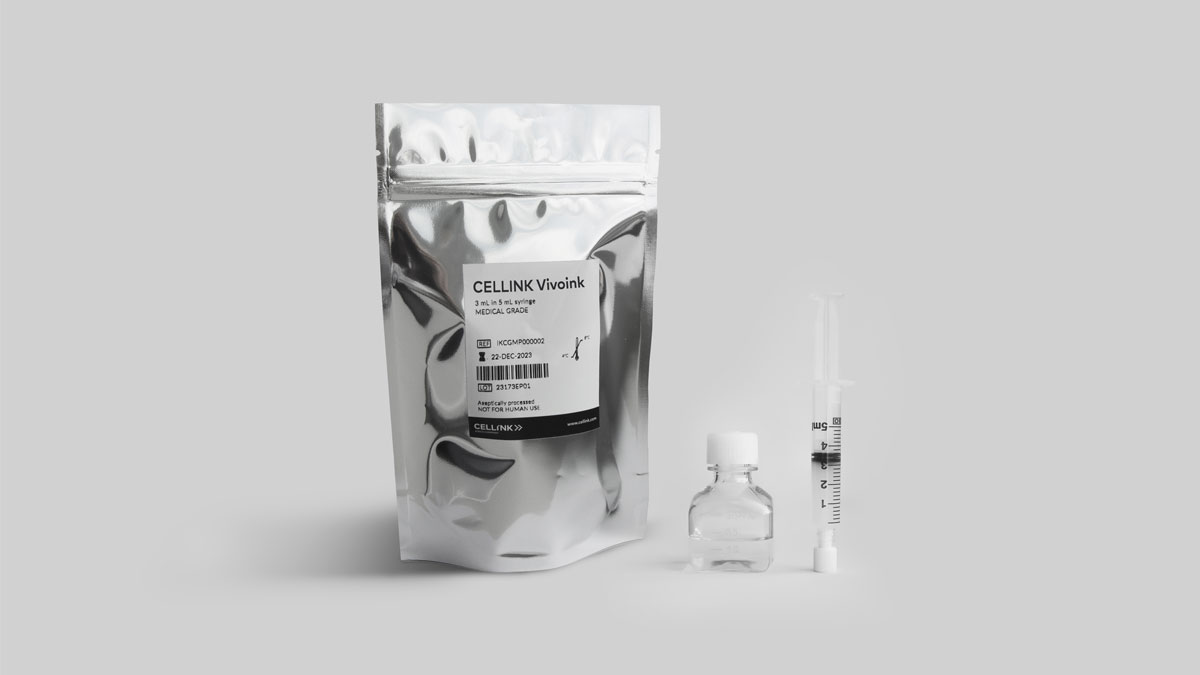
Gothenburg, October 18th 2023 — CELLINK, a global leader in bioprinting technologies, is proud to announce a pioneering innovation in the field of regenerative medicine and tissue engineering with the launch of CELLINK Vivoink, the first-ever medical-grade bioink specially designed to support researchers on their clinical translational journey. CELLINK Vivoink is optimized for superior printability, mechanical stability, and cell viability, making it a game-changer in the bioprinting industry.
Cell-based therapy and tissue engineering have long held the promise of transforming healthcare by enabling the regeneration of damaged tissues and the development of advanced disease models. However, the lack of bioinks suitable for clinical applications has been a significant barrier to progress. CELLINK’s latest breakthrough, CELLINK Vivoink, addresses this challenge, providing researchers with a bioink that can fulfill the regulatory requirements for clinical translation.
The product marks another important step in CELLINK’s strategic commitment towards providing solutions that enable the future of health. Produced under stringent quality control, with selected manufacturing requirements from ISO 13485 and GMP Annex 1 certification for production facilities, CELLINK is providing users with a batch-to-batch material consistency vital for translation to clinics.
“CELLINK Vivoink represents a major milestone in bioprinting technology,” said Cecilia Edebo, CEO of CELLINK. “We are proud to be at the forefront of innovation in the field of regenerative medicine and tissue engineering, and we believe that CELLINK Vivoink will empower researchers to take significant steps toward clinical applications of bioprinting, bringing us closer to realizing the promise of bioprinting and enabling our valued customers to create the future of health.”
Building on the formulation that allowed many researchers to successfully publish breakthrough results, CELLINK Vivoink ushers in a new era of quality that can spur on scientific discovery even further. “We have focused our development efforts to elevate CELLINK Vivoink in terms of consistent printability, mechanical stability and cell viability. With expertly chosen base materials, stringent manufacturing and rigorous quality control, CELLINK Vivoink truly provides researchers with the ideal material to translate their research from the benchtop to the bed side”, said Itedale Namro, CELLINK CSO.
For more information about CELLINK Vivoink, please visit the product page here.
For further information, please contact:
Avijit Minocha, Head of Marketing, CELLINK
Phone: +1 617 637 5372
Email: akm@cellink.com
About CELLINK
CELLINK is enabling the future of health as part of BICO, the world’s leading bioconvergence company. When CELLINK released the first universal bioink in 2016 and subsequently launched the world’s first affordable 3D bioprinter it democratized the cost of entry for researchers around the world and played a major role opening up the field of 3D bioprinting. Today, the company’s best-in-class bioinks, bioprinters, software and services have been cited in over 1000 publications and are trusted by more than 1,600 academic, pharmaceutical and industrial labs. With a comprehensive portfolio of world-class 3D bioprinters and bioinks CELLINK’s technology enables the printing of human tissues and organs, which enable faster and more accurate models for drug development, while replacing animal experiments and paving the way to save lives by reducing organ rejection and potentially solve the problem with lack of donors.