Engineering a 3D human myocardial tissue model
Products used:
Aim
To assess the application of CELLINK GelMA, GelXA, and GelXA LAMININK 521 (in graphs shortened GelLAM) a 3D human in vitro model of myocardial tissue was engineered. Induced pluripotent stem cells (iPSC) were differentiated into cardiomyocytes (CMs) and combined with human fetal cardiac fibroblasts (hfCF) in the hydrogels. These were used to fabricate 3D microtissues to establish a co-culture model.
The G-code to this three-layered grid structure can be found on Bioverse!
Increasing cell viability
iPSC-CM in co-culture with hfCFs in a near-native concentration were mixed into GelMA, GelXA, or GelXA LAMININK 521 and cultured for 11 days at 37°C and 5% CO2. Two different photocrosslinking times were evaluated; 30 and 60 seconds with a 405 nm module.
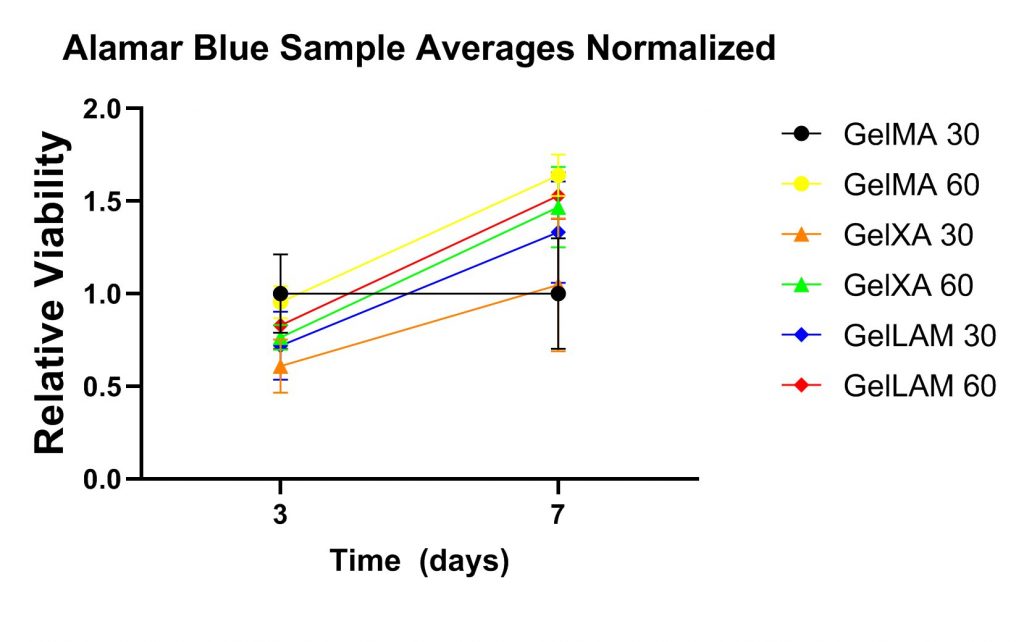
An Alamar Blue assay was employed to assess cell viability and an increase in cell viability seven days compared to three days after bioprinting was observed across all conditions, displayed in the adjacent figure.
Constructs match human beating rates
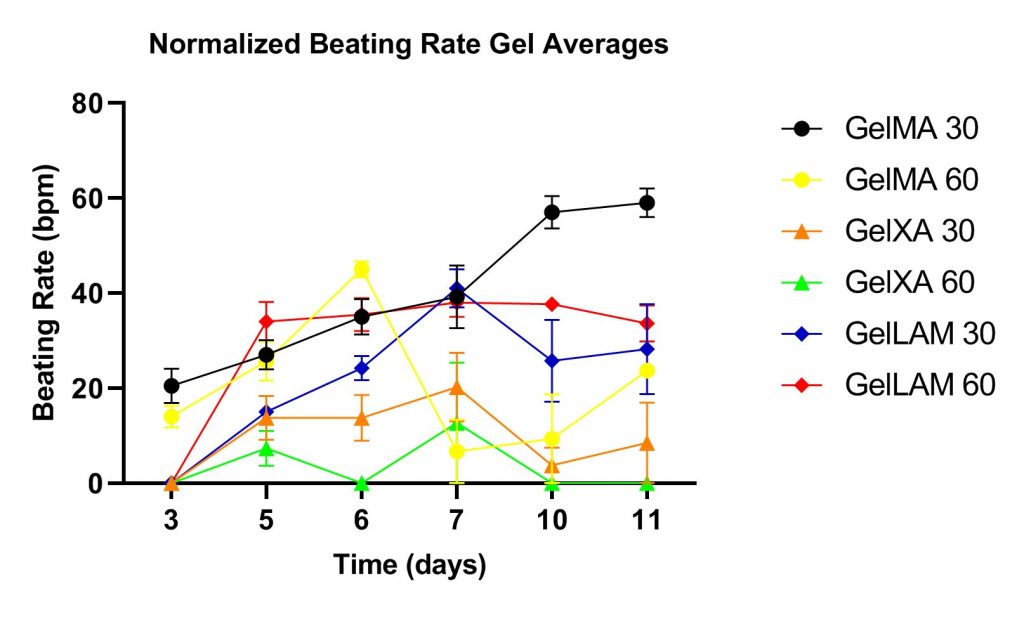
Synchronized contractions
Interestingly, the GelXA LAMININK 521 and GelMA groups displayed spontaneous uniform, synchronised contractions, resembling functionality in the native myocardial tissue. The contraction of the GelXA LAMININK 521 at day seven can be seen in the adjacent video. Synchronous contractions are indicative of cardiomyocyte networking, enabling intracellular communication and transfer of action potentials to neighboring cells. The GelXA groups did not display these kinds of contractions during the experiment. The contractions observed in the GelXA 30 s and 60 s were asynchronous between multiple clusters of contracting cells. This indicates that the GelXA gels were not sufficiently re-arranged by the hfCFs to allow coupling of cardiomyocytes.
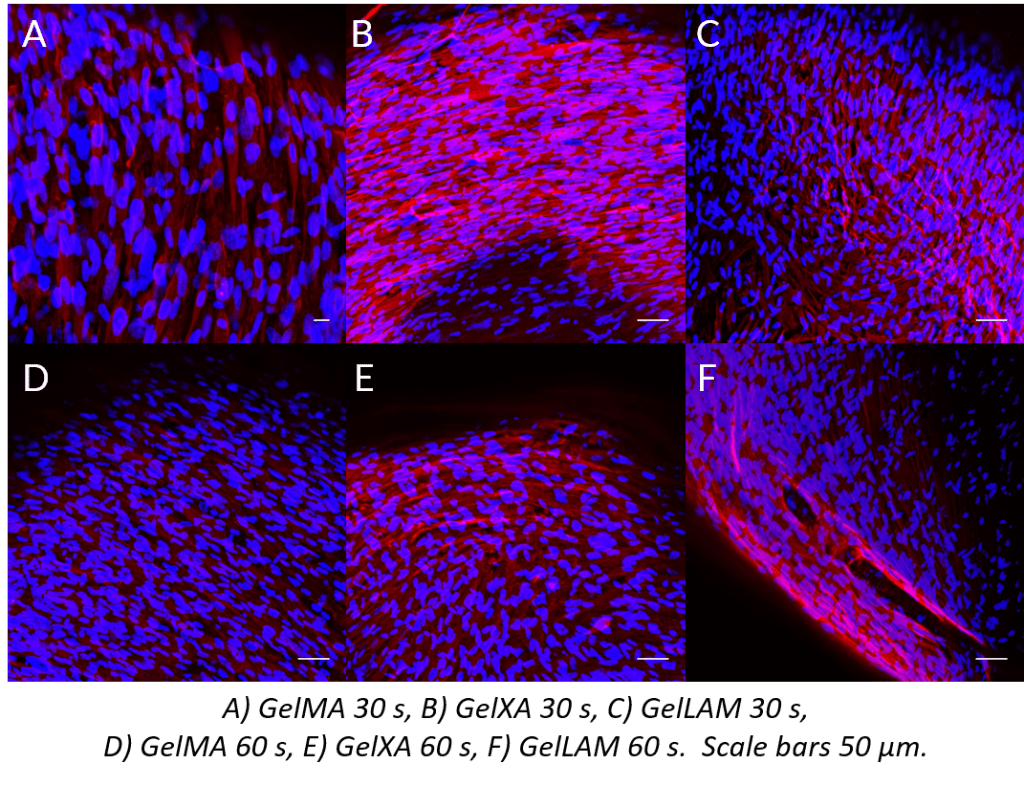
Parallell cell alignment
Finally, constructs were stained with Hoechst nuclear stain and a Vimentin antibody. The adjacent figure visualizes iPS-CMs and hfCFs with Hoechst nuclear stain (blue) and vimentin (red) in hfCFs in A) GelMA 30 s, B) GelXA 30 s, C) GelLAM 30 s, D) GelMA 60 s, E) GelXA 60 s, F) GelLAM 60 s, scale bars 50 µm. In all constructs, parallel alignment of cells is observed.
Conclusion
This preliminary data show that both GelMA and GelXA LAMININK 521 are promising candidates in establishing a 3D human myocardial tissue model. Synchronous, uniform contractions at rates resembling the resting heart rate in humans were observed in these groups. Further fine-tuning of printing parameters and cell culture conditions will hopefully lead to a reproducible co-culture model that can be used as a foundation for tissue engineering applications and for developing a high-throughput model for drug testing.





