University of Pavia Collaboration
3D model muscle fiber
Products used:
CELLINK FIBRIN ➝
INKREDIBLE+
Aim
University of Pavia
The present research activity about 3D bioprinting of muscle cells is carried out at University of Pavia (Italy) by the collaboration of two research groups.
CompMech Group, led by Prof. F. Auricchio, has a strong experience in advanced materials, solid and structural mechanics, computational simulations but also in 3D printed materials and additive manufacturing technologies. Within the group, Eng. F. Scocozza, Dr. M. Conti and Dr. S. Marconi are dealing with bioprinting.
Regenerative Medicine Lab is a research group located at the University of Pavia. The group, led by Prof. Gabriella Cusella and Prof. Maurilio Sampaolesi, is focused on tissue engineering strategies for the regeneration of mesodermal tissues, in particular bone, dermis, skeletal and cardiac muscle. The competences of the team cover the complete spectrum of expertise necessary to move from stem cell in vitro culture and research to bio-complexes involved in tissue repair. Within the group, Dr. Flavio Ronzoni, Dr. Gabriele Ceccarelli and Laura Benedetti are working with bioprinting.
The present research activity is supported by the two University of Pavia flagship projects: CHT (Centre for Health technologies) and 3D@UniPV (Virtual Modeling and Additive Manufacturing for Advanced Materials).
Bioprinting of myoblasts
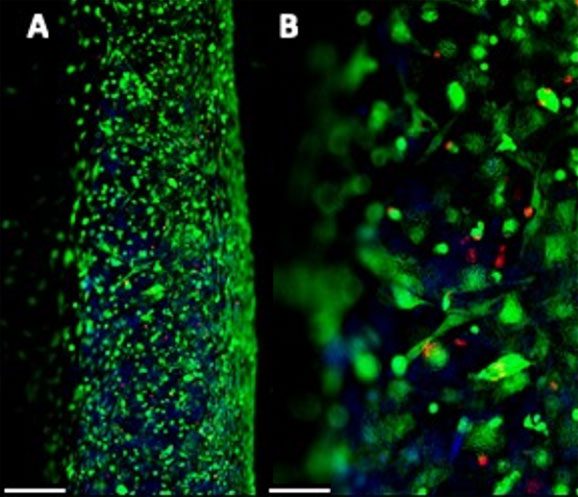
Cell viability
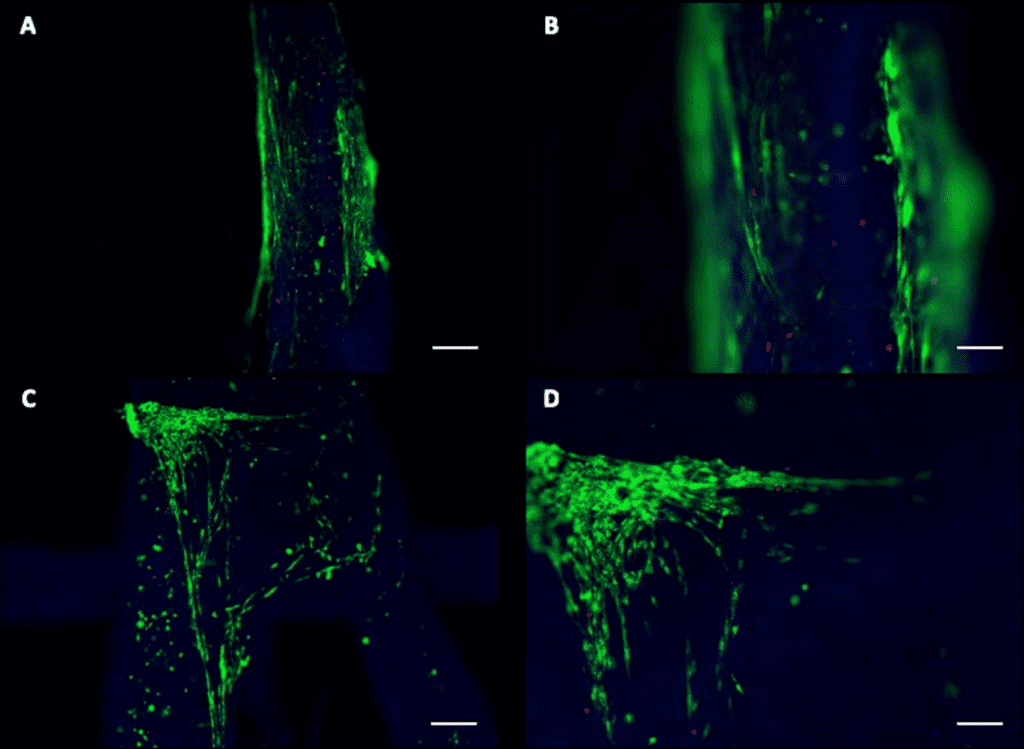
Results
The results from the bioprinting of C2C12 myoblast with the CELLINK’s Fibrinogen bioinkshow:
- Uniform cell distribution, optimal printability and good cell viability up to 21 days.
- Better cell differentiation on crosslinked edges.
- Live/Dead Assay shows myotubes formation.
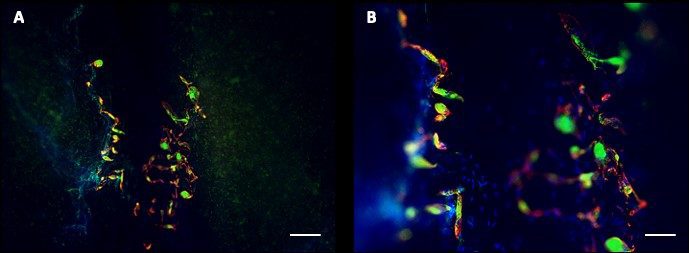
- Immunofluorescence analysis after 27 days of differentiation shows good formation of both actin and myosin filament, shown beside.
- Expression of two important master genes related to muscle differentiation (MyOD and MCK) highlight a positive effect of the 3D construct on the differentiation process.