Quality Control
Commitment to quality
The best bioinks come from the best sources of raw material. We select suppliers based on our strict standards, and we test each delivery for endotoxins and sterility. From the forests of Sweden to the cold waters of Norway, we strive to source the best raw ingredients and deliver the maximum value possible to our customers.
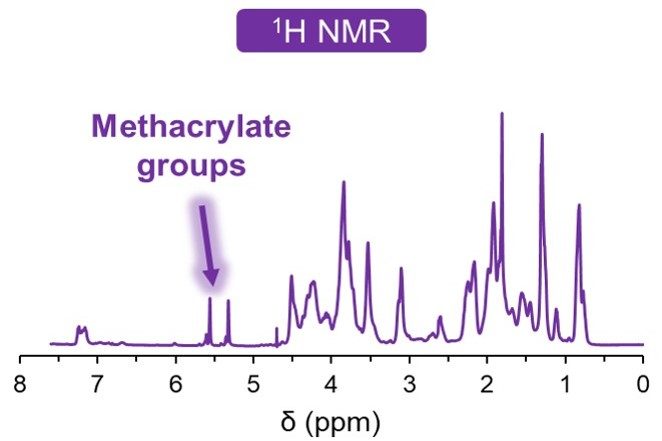
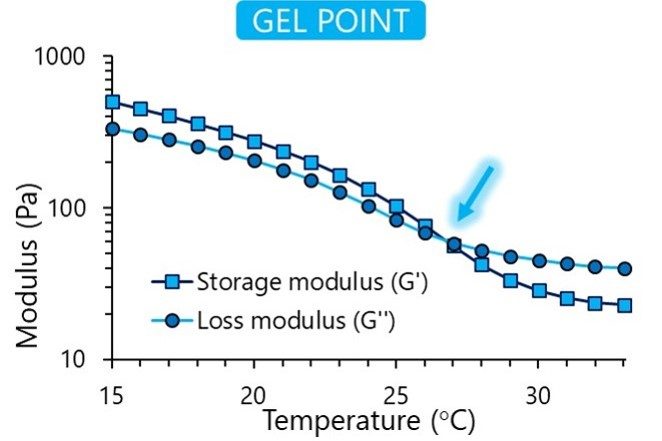
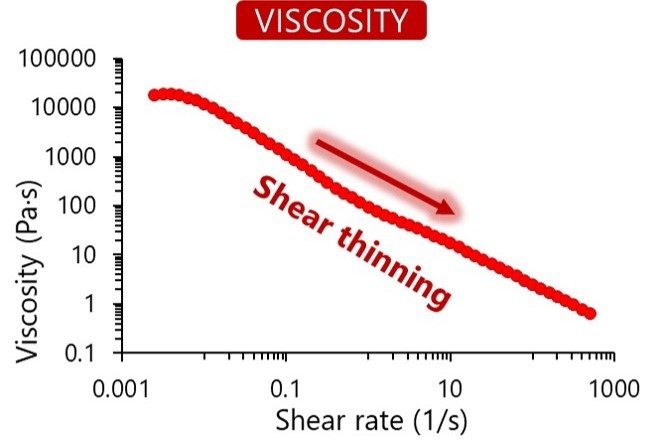
Once the raw materials are approved, they enter our sterile and aseptic production process. This clean area ensures toxin-free manufacturing. We understand the importance of consistency in your research, and each final product is controlled for quality to guarantee reproducibility. We verify each bioink’s viscosity, appearance, pH and cytotoxicity, and in addition the gel point and degree of methacrylation for GelMA-based bioinks.
In our online store, you can find specifications for each bioink listing the results from our quality control process. The figures below show the typical results of the tests we perform.
Sterility
Endotoxin level
pH
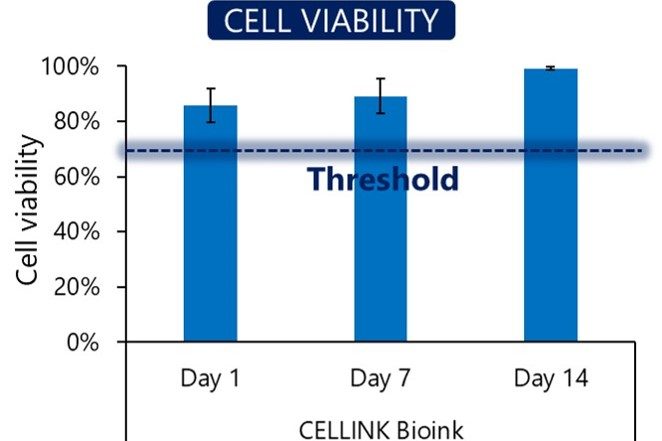
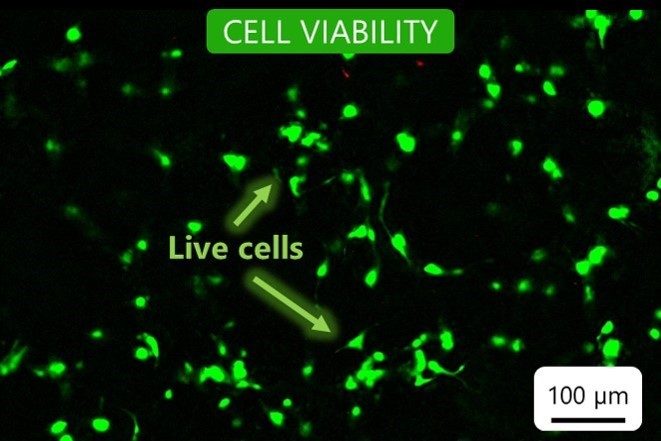
Cell viability
Our bioinks contain no cytotoxins. To ensure this, we test cell viability after seven days of culture in a bioprinted construct. We confirm that the cells grow and proliferate in the bioink environment.





