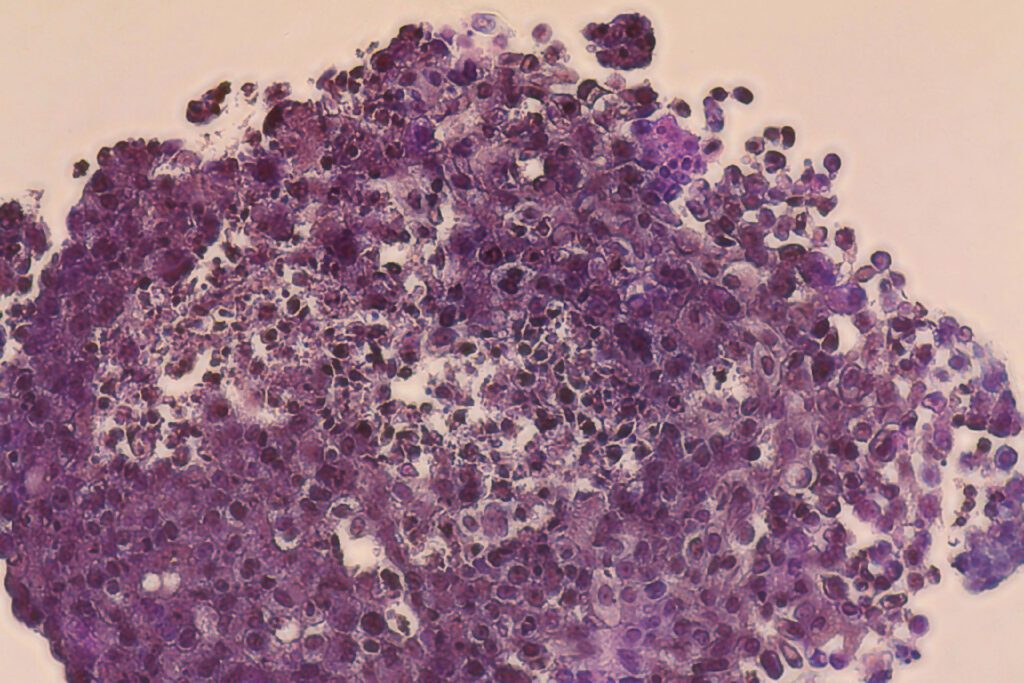
Ishani Malhotra is CEO and founder of Carcinotech, the Edinburgh-based biotech that is 3D bioprinting breast cancer models for drug testing with cells from biopsy samples. Find out how she and her ingenious researchers benefited from expert support from CELLINK, a BICO company.