- May 5, 2021
Mending Hearts – On-demand Bioprinted Patient-specific Heart Tissue
Dr. Carmine Gentile’s lab in Sydney is developing 3D bioprinted heart tissue with patient cells to solve organ donor shortage and minimize the risk of transplant rejection.
The leading cause of death globally is heart disease
Patients all over the world wait on long heart transplant lists. A contributing factor for the shortage is that modern medicine can’t repair damaged heart muscles after heart attacks. Even the lucky few who find a matching donor, face more hurdles, including organ viability, geographical distance, immune rejection. As a result, heart disease remains the leading cause of death worldwide. Each year, cccording to the World Health Organization, 17.9 million lives are lost to cardiovascular diseases.

“We are using the patient’s stem cells so that there’s no risk of rejection.”
— Dr. Carmine Gentile, whose lab at UTS is working on 3D bioprinted heart tissue from patient cells
3D bioprinting heart tissue
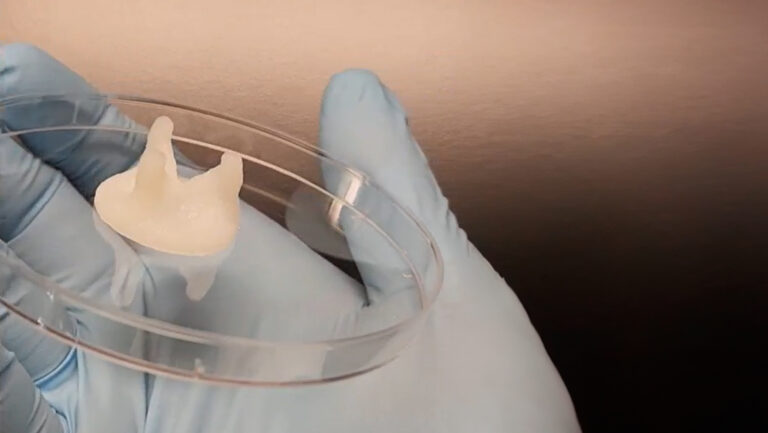
Dr. Carmine Gentile, leader of the Cardiovascular Regeneration Group at the University of Technology Sydney (UTS), announced in 2020 that his lab had “developed a technology that can 3D model and bioprint personalized hearts for transplantation, using the patient’s own stem cells so that there’s no risk of rejection.” Specifically, his research identified the optimal conditions to get stem cells to produce blood vessels in the 3D bioprinted heart patches.


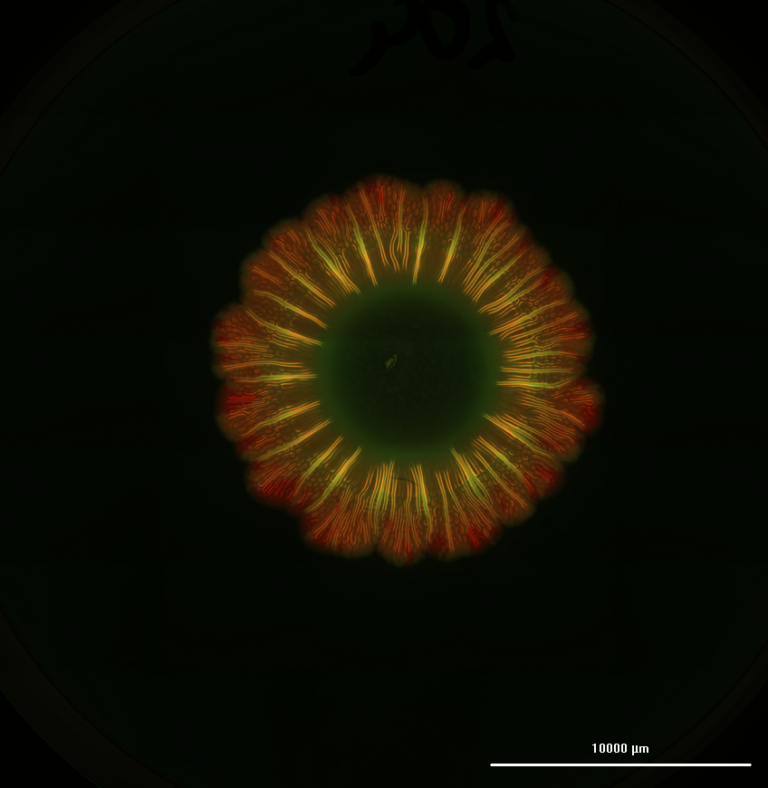
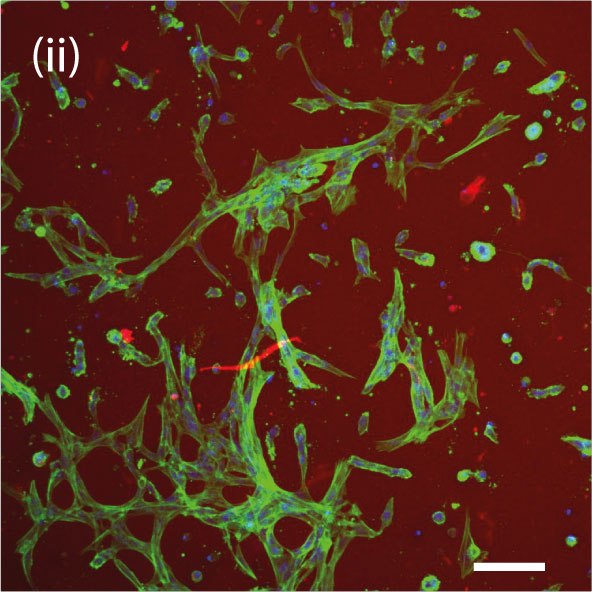
In the 2020 study, the lab bioprinted murine cells on CELLINK’s BIO X™ using an alginate-gelatin hydrogel. These bioprinted cardiac cells were allowed to culture for 7 to 14 days until they developed into beating patches of heart tissue. During open-heart surgery on the mouse, the 3D bioprinted vascularized heart patch was transplanted to a damaged area of the heart. The transplant was meant to test the safety of the 3D bioprinted heart patch and to ensure it had a positive effect on heart function. Both were verified.


Optimizing 3D bioprinted heart tissue
“This could be a game changer,” says Chris Roche, a cardiothoracic surgical trainee and PhD candidate at UTS, who co-authored the 2020 study with Dr. Gentile, his PhD advisor. With additional support from the University of Sydney, the Catholic Archdiocese of Sydney and Heart Research Australia, Roche’s doctoral thesis seeks to further optimize these vascularized bioprinted heart patches as well as the transplanting procedure. His goal is to one day reach clinical trials on human patients. “It would mean on-demand replacement of heart cells after a heart attack with no chance of rejection, less risk of heart failure and, importantly, no waiting list for a heart transplant, ” says Roche optimistically.
Creating the future of medicine
“We know patients are in urgent need of a safe alternative to heart transplants,” says Dr. Gentile. “We’re working hard to further develop our technology and make sure it can be available in the shortest time possible.” Dr. Gentile’s lab will continue focusing on the in vivo testing of the 3D bioprinted heart tissue and fine-tuning the protocols. One thing is certain, CELLINK will be there to support this enterprising lab’s work, allowing them to one day bioprint human heart tissue for patient-specific regenerative medicine and, thus, create the future of medicine.
Further reading
UTS Profile of Dr. Carmine Gentile
Roche CD, Brereton JR, Ashton AW, Jackson C, Gentile C. European Journal of Cardio-Thoracic Surgery. 2020. DOI:10.1093/ejcts/ezaa093.
Roche CD, Gentile C. Journal of Visualized Experiments. 2020. DOI:10.3791/61675.
Roche CD, Sharma P, Ashton AW, Jackson C, Xue M, Gentile C. Frontiers in Bioengineering and Biotechnology. 2021. DOI:10.3389/fbioe.2021.636257.