- June 13, 2022
How 3D Bioprinted Wound-healing Patches Deliver Antibiotics and Better Outcomes for Diabetic Foot Ulcer Patients


“We want to make treatment less painful and time-consuming for DFU patients with drug-loaded 3D bioprinted wound-healing scaffolds,” says Professor Dimitrios Lamprou of Queen’s University Belfast.
How a family member’s experience with diabetic foot ulcers inspired Prof. Lamprou’s 3D bioprinted wound-healing patch
The BIO X is helping accelerate the healing of diabetic foot ulcers (DFU) with 3D bioprinted patches that enable sustained release of antibiotics directly to the wound area. For the enterprising Lamprou Lab in Belfast, this breakthrough is more than academic; you could say it is also personal.
It seems Professor Dimitrios Lamprou, the chair of Biofabrication and Advanced Manufacturing at the School of Pharmacy, Queen’s University Belfast, was destined for this project. He has always been an early adopter of emerging technologies, including 3D printers and bioprinters. In fact, over the past 4 years, his lab has worked on several 3D printed antibiotic scaffolds. But after seeing a family member undergo treatment for a DFU, it was a no-brainer that his next project would focus on improving the treatment of DFU wounds.
3D bioprinted patches prevent bacterial infections that interfere with proper wound healing in diabetic foot ulcer patients
More than a third of diabetics develop DFUs in their lifetime, and nearly half of those wounds get infected, with Staphylococcus aureus, Pseudomonas and Escherichia coli being the most common pathogens. These infections contribute to poor wound-healing outcomes for diabetic patients and could even lead to lower-limb amputations. “I wanted to make DFU treatment less painful and time-consuming,” says the professor of pharmacy.
Why extrusion-based 3D bioprinting and the BIO X were chosen to fabricate the biodegradable wound-healing patches
After experimenting with inkjet-based and laser-assisted biofabrication options, the team concluded that extrusion-based 3D bioprinting was the most promising technology in the context of DFU treatment. One distinct advantage with extrusion is the flexibility to use a wide range of biomaterials in one print. According to Professor Lamprou, the “straightforward” BIO X extrusion-based 3D bioprinter is also a game changer when it comes to setting printing parameters at the individual printhead level. He adds, “You could say the BIO X is foolproof because it is so easy to use.”
The FDA-approved polycaprolactone polymer proved to be the ideal biodegradable biomaterial for the wound-healing patch
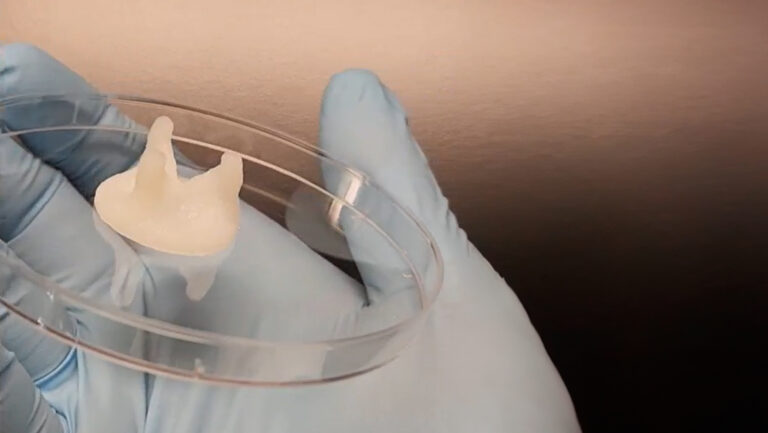
The paper in Drug Delivery and Translational Research details how the BIO X bioprinted a mix of the biodegradable polymer polycaprolactone (PCL), which has been previously cleared by the FDA for use in other biomedical devices, with the broad-spectrum fluoroquinolone antibiotic levofloxacin to produce the wound-healing scaffolds. “These thermoplastic hydrogels are excellent for wound treatment given their biocompatibility, slow rate of degradation and good mechanical properties, such as flexibility to move freely,” explains Professor Lamprou.
At the patient level, Lamprou Lab’s antibiotic delivery patches solve several concerns. For example, the higher doses needed for oral administration of antibiotics can induce toxicity, while intravenous administration can be invasive, even requiring hospitalization. Not to mention reducing the proliferation of antibiotic-resistant bacterial strains attributed to the overuse of system-wide antibiotics. The slow degradation of PCL approach, however, facilitates the sustained, localized delivery of antibiotics at the wound site, provides a robust support structure as the skin repairs itself, and doctors can easily dress a wound in their office with no need for costly hospitalization.
CELLINK’s support team saved the day when the Belfast lab needed them most
Professor Lamprou is quick to add that CELLINK’s support team was invaluable. “The truly knowledgeable support team was there to answer our questions and offer practical suggestions, especially when it came to choosing polymers, making adjustments for different molecular weights and setting parameters. They saved us a lot of time,” the professor acknowledges. “To be honest, the level of service is unique. In one instance, when we had to send out our BIO X for maintenance, CELLINK went above and beyond by lending us the local sales office’s demo model to avoid delaying our experiments! You don’t hear of many companies doing this.”
Keep an eye out for more biomedical breakthroughs from the Lamprou Lab
“With modifications, our bioprinted drug-loaded scaffolds could potentially work for bone cancer, melanoma and also burns,” says Professor Lamprou. “So far, we have published only the results of the scaffolding and antibiotics. Once we have secured the relevant patents, we will publish more exciting discoveries related to incorporating additional biologics in the bioprinting to support the regrowth of tissue.”